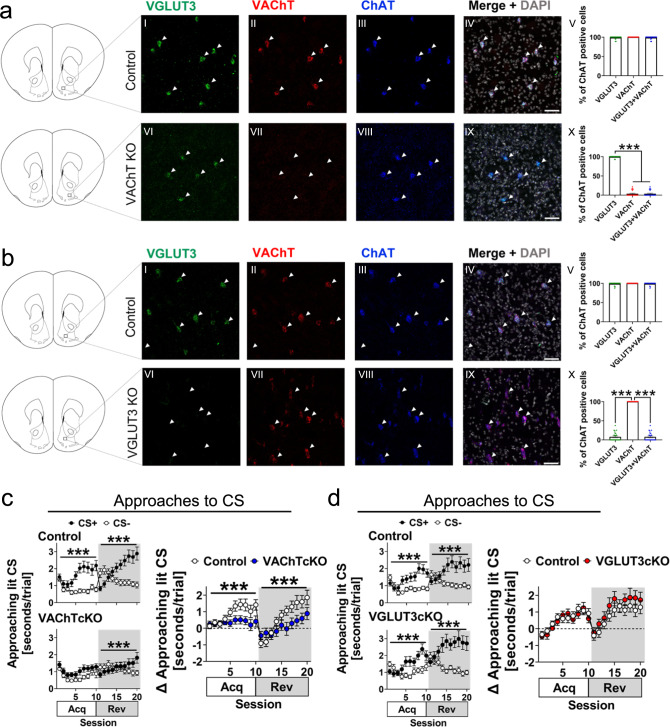
Fig. 3. Acetylcholine release from cholinergic interneurons, but not glutamate, is required to regulate approach behaviours toward reward-predicting cues.
a (Top) Triple-fluorescence in situ hybridisation (RNAscope) revealed that mRNA transcripts for the vesicular glutamate transporter type 3 (slc17a8, VGLUT3-green), the vesicular acetylcholine transporter (slc18a3, VAChT-red), and the choline acetyltransferase transporter (chat, ChAT-blue) simultaneously express in interneurons from the nucleus accumbens of control littermate mice (N = 3 VAChTflox/flox mice, n = 24 cells/mouse). The boxed area from the depicted coronal brain section is enlarged in the right panels (I–IV) showing cholinergic interneurons abundantly expressing VGLUT3, VAChT and ChAT (arrowheads); nuclei were stained with DAPI (grey). (V) All ChAT+ cholinergic interneurons co-expressed transcripts for VGLUT3 and VAChT (p > 0.05). (bottom) In contrast, VAChTcKO mice (N = 3 mice, n = 24 cells/mouse; panels VI–IX) did not express transcripts for VAChT (VII), despite (X) VGLUT3 and ChAT mRNA were visualised in cholinergic interneurons (arrowheads) (one-way ANOVA, F(2,66)=2065, p < 0.0001). Scale bar–50μm. b (Top) Nucleus accumbens neurons from control littermate mice showed abundant expression of VGLUT3 (green), VAChT (red) and ChAT (blue) transcripts (N = 3 VGLUT3flox/flox mice, n = 24 cells; panels I–IV). Nuclei were labelled with DAPI (grey). (V) All ChAT+ cholinergic interneurons also expressed VGLUT3 and VAChT mRNA. (bottom) No VGLUT3 transcript (arrowheads) was observed in neurons expressing VAChT and ChAT transcript in VGLUT3cKO mice (N = 3, n = 24 cells/mouse; panels VI–X). One-way ANOVA, F(2,69) = 830.8, p < 0.0001. Scale bar–50μm. c In contrast to control littermate mice (N = 24 VAChTflox/flox mice, n = 13♂, n = 11♀), VAChTcKO mice (N = 25, n = 11♂, n = 14♀) performing the Autoshaping task showed deficits to approach the CS+ during acquisition (Acq) and reversal (Rev) sessions (two-way RM-ANOVA SessionXCS interaction, Acq-VAChTcKO: p > 0.05; Rev-VAChTcKO: F(9,432) = 7.197, p < 0.0001; Acq-Control: F(9,414) = 8.299, p < 0.0001; Rev-Control: F(9,414) = 17.96, p < 0.0001). Moreover, the relative time (Δ) mice approached reward-predicting CS revealed that VAChTcKO (blue circles) were impaired when compared to control littermate mice (blank circles). Two-way RM-ANOVA SessionXGenotype interaction, Acq: F(9,423) = 3.203, p = 0.0009; Rev: F(9,423) = 4.232, p < 0.0001). d VGLUT3cKO mice (N = 23, n = 12♂, n = 11♀) spent more time approaching the CS+ across acquisition and reversal sessions, similar as their control littermate mice (N = 24 VGLUT3flox/flox, n = 12♂, n = 12♀). Mixed-effects model SessionXCS interaction, Acq-VGLUT3cKO: F(9,394) = 7.212, p < 0.0001; Rev-VGLUT3cKO: F(9,395) = 9.091, p < 0.0001; Acq-Control: F(9,414) = 6.897, p < 0.0001; Rev-Control: F(9,414) = 10.68, p < 0.0001. The relative time (Δ) mice approached reward-predicting CS showed that VGLUT3cKO (red circles) were similar than control littermate mice (blank circles) across sessions (two-way RM-ANOVA SessionXGenotype interaction, Acq: p > 0.05; Rev: p > 0.05). Post-hoc Tukey’s test: ***p < 0.0001, **p < 0.001, *p < 0.05. No adjustments were made for multiple comparison analyses. Data are presented as the mean ± SEM. Source data are provided as a Source Data file.