Abstract
Purpose of Review
There is little doubt that the consensus has changed to favor preservation of meniscal function where possible. Accordingly, the indications for meniscal repair strategies have been refocused on the long-term interest of knee joint health. The development and refinements in surgical technique have been complemented by biological augmentation strategies to address intrinsic challenges in healing capacity of meniscal tissue, with variable effects.
Recent Findings
A contemporary approach to meniscal healing includes adequate surgical fixation, meniscal and synovial tissue stimulation, and management of the intraarticular milieu. Overall, evidence supporting the use of autogenous or allogeneic cell sources remains limited. The use of FDA-approved medications to effect biologically favorable mechanisms during meniscal healing holds promise.
Summary
Development and characterization of biologics continue to advance with translational research focused on specific growth factors, cell and tissue behaviors in meniscal healing, and joint homeostasis. Although significant strides have been made in laboratory and pre-clinical studies, translation to clinical application remains challenging. Finally, expert consensus and standardization of nomenclature related to orthobiologics for meniscal preservation will be important for the advancement of this field.
Keywords: Meniscus, Meniscal repair, Biologics, Biological augmentation, Drug repurposing
Introduction
The menisci function biomechanically by guiding load distribution, providing joint congruency, acting as a shock absorber, and enhancing knee stability. Biologically, the menisci contribute to joint lubrication and articular cartilage nutrition and likely serve an active role in overall joint homeostasis [1]. Considered together, the combined biomechanical and biological functions of the menisci serve an overall chondroprotective role [2]. Historically, the clinical association between osteoarthritis and meniscectomy was first established by Fairbanks in 1948 and iterative scientific developments have prompted a shift to preserving the meniscus where possible [2–4]. As a direct result, advances in surgical fixation techniques and biological augmentation strategies have been applied to improve outcomes and to expand the indication of meniscal repair.
From a societal perspective, the burden of meniscal pathology is significant with over 1,000,000 meniscal procedures performed in the USA annually, and this number is growing [5]. Unfortunately, clinical results after isolated meniscal repair are not always satisfactory and have been aggregated to an overall failure rate of around 25% in the literature; this may be at least partially due to extending the indications to tears with poor intrinsic healing potential in an attempt to salvage the meniscus [6•]. Specific tear and patient characteristics remain critical when indicating a meniscal repair and assessing the efficacy of repair and augmentation strategies [7, 8]. Contraindications to repair include poor tissue vascularity, degenerative tissue changes, advanced patient age, poor patient compliance with rehabilitation, and the presence of either knee instability or osteoarthritis. Magnetic resonance imaging (MRI) is important for characterizing both the tear pattern and tissue quality. Tear characteristics currently deemed unsuitable for repair include chronicity of the tear, smaller longitudinal tears (< 10mm), radial tears limited to the inner two-thirds of the meniscus (avascular region), and degenerative tears [2]. From a biological standpoint, vascularity of the repair influences outcome with peripheral, well-vascularized “red–red” zone tears having the best propensity for healing, followed by the “red–white” junction. Tears in the inner avascular “white–white” zone have the least propensity to heal [9••, 10]. Regarding precise technique, contemporary “all-inside” repair techniques demonstrate comparable outcomes to inside-out repair, including peripheral unstable longitudinal “bucket-handle” tears [11].
The term “biologics” is generally accepted to include techniques that employ the selective concentration of patient-derived peripheral blood and the harvesting of autogenous “progenitor cells” from the bone marrow and adipose tissue. Allogeneic biological treatments include the utilization of tissue or cells derived from the placenta, amnion, and fetal umbilical vein [12]. In general, biologics have been applied in the setting of meniscal repair to enhance meniscal tissue healing and to manage the post-injury and post-surgery joint milieu.
Biological targets to enhance meniscal healing include (i) cell homing from the synovium and surrounding meniscus, (ii) stimulation of intrameniscal cell proliferation and matrix production, (iii) increased vascularity at/to the repair site, and (iv) stimulation of matrix synthesis and remodeling of the repair tissue. Simultaneously, management of the post-injury and post-surgical inflammatory joint milieu attempts to create an intra-articular environment that is more conducive to meniscal healing by normalizing protease and cytokine activity to restore joint homeostasis and ultimately improve long-term knee joint health. These considerations suggest that the nature and timing of biologics applied to meniscal treatment vary significantly and that an optimized, comprehensive approach likely warrants multiple strategies over multiple time points.
Focusing on meniscal tissue repair, the role of specific growth factors has been demonstrated in vitro for important cell behaviors including cell migration, proliferation, and matrix production [1]. In vivo, fibroblast growth factor-2 (FGF-2) and connective tissue growth factor (CTGF) have demonstrated enhancement of meniscal repair in a rabbit model, and vascular endothelial growth factor (VEGF) was effective in a sheep model [13–15]. While specific growth factors have been applied to enhance bone healing, they are yet to be applied clinically for meniscal healing. Nonetheless, an understanding of the effect of specific growth factors may guide the development of optimal biological strategies using quantitative compositional characterization in the context of objective and subjective clinical outcomes [16].
The purpose of this review is to summarize recent advancements in biologics applied to meniscal injury and treatment. These developments are discussed thematically with an emphasis on translational and clinical data.
Physical Modalities at the Tear Site: Meniscal and Synovial Tissue Stimulation
The application of mechanical techniques to stimulate healing around the repair site by stimulating vascular ingrowth into the outer meniscus and adjacent synovium represents the earliest forms of biological augmentation. The rationale for synovial abrasion is to stimulate the synovial reflection present at the peripheral attachment of the medial and lateral menisci on the femoral and tibial articular surfaces to optimize the reactive pannus formed following injury. Ochi et al. demonstrated that hypertrophic synovium expresses favorable cytokine profiles in the rabbit model [17]. Arnoczky et al. demonstrated that synovial abrasion resulted in enhanced fibrovascular healing due to proliferation of vascularized synovial tissue at the repair site in the dog model [18]. A study by Nakhostine et al. made the distinction that the technique was less effective on tears further from the synovial rim [19]. Overall, clinical data supporting synovial abrasion alone and in conjunction with other augmentation strategies for the treatment of stable or partial thickness meniscal tears have helped popularize this technique despite the lack of control groups, and its simplicity and relative ease of execution have made it a popular technique during meniscal repair (Fig. 1).
Fig. 1.
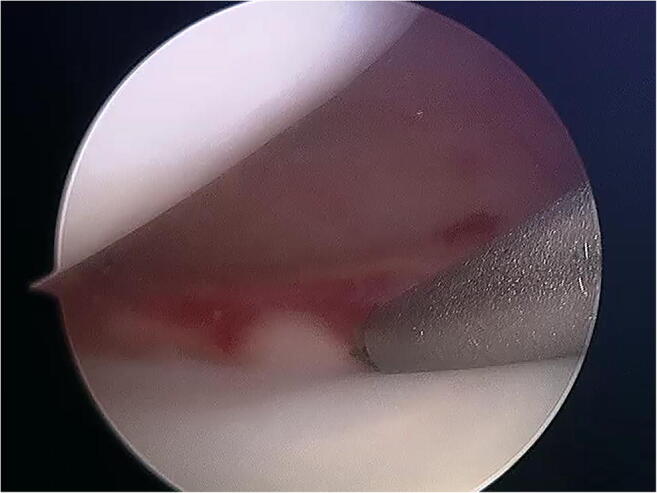
Synovial abrasion is performed using a synovial rasp at the repair site
The technique of trephination was introduced as a means of creating a vascular access channel while avoiding significant damage to the collagen architecture of the meniscus. A series of horizontally orientated trephinations are made with a hypodermic needle (18-gauge needle or larger) to produce a series of bleeding puncture sites at the peripheral aspect of the meniscal rim, which provides an avenue for vascular in-growth. Fox et al. reported this technique in the treatment of incomplete lesions in the peripheral and middle third of the meniscus reporting a 90% success rate in their series that lacked a control group [20]. Zhang and Arnold compared 36 patients who underwent suture fixation of a meniscal tear with trephination to 28 patients with suture repair alone [21]. They observed a significantly smaller number of symptomatic retears in the trephination group, concluding that trephination was a safe, expedient, and efficacious augment to meniscal repair.
Interfacial Tear Treatments
The use of exogenous fibrin clot to augment meniscal repair was initially developed in animal models and has translated to patients with some clinical success, despite potential challenges in maintaining the clot in situ at the repair site [22, 23]. Blood clots contain various cytokines (vascular endothelial growth factor, insulin-like growth factor-1, fibroblast growth factor basic, hepatocyte growth factor, and stromal cell-derived factor-1) known to stimulate meniscal cells in vitro with some evidence suggesting that fibrin clots derived from the bone marrow contain greater quantities than those from peripheral blood [24]. Fibrin clot preparation from peripheral blood takes 3 to 5 min and requires 30 mL for a 1–2-mL clot; this is placed in a sterile beaker and then using a sintered glass barrel from a 20-mL glass syringe, the blood is gently stirred gently until a fibrin clot is precipitated at the surface. The final consistency of the fibrin clot resembles wet chewing gum and can hold a suture (Fig. 2). Henning et al. reported positive results using a fibrin clot injected into the seam of the repair with an 8% failure rate versus 41% failure rate in repairs without fibrin clot [25]. Similar positive results were seen in a subsequent study from the same group using a fascial sheath to contain the clot at the repair site of complex tears [26]. Similarly, Yamanashi et al. described a technique using a polyglycolic acid (PLGA) sheet to contain the blood clot at the repair site [27]. Van Trommel et al. described a series of five radial tears of the lateral meniscus that extended to the popliteus treated with fibrin clot augmentation that all went on to heal based on direct evaluation with second-look arthroscopy [28]. Similarly, Ra et al. retrospectively reviewed patients with complete radial tears (9 lateral, 3 medial) who underwent inside-out repair with fibrin clot; 11/12 showed evidence of complete healing on MRI with 6/7 seen to heal on second look arthroscopy with associated improvements in clinical outcome metrics [29]. Jang et al. reported a success rate of 95% in 41 meniscus tears (19 radial tears, 12 longitudinal tears in the red–white zone, 7 transverse, and 3 oblique) following augmented arthroscopic inside-out repair with autologous fibrin clot [30]. Kamimura et al. described a series of 10 patients with degenerative horizontal cleavage tears that were repaired using fibrin clot, with 70% healing on second look arthroscopy [31].
Fig. 2.
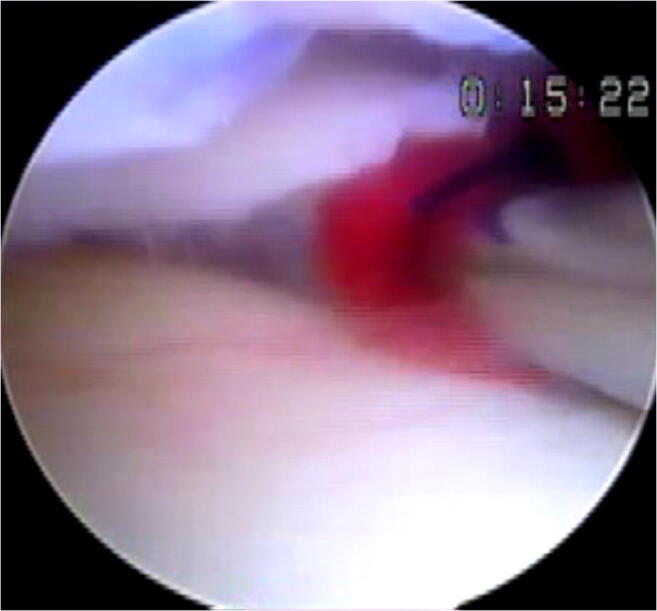
A clot is maintained at the tear site and incorporated into the repair
Platelet-rich fibrin (PRF) is a blood product with structural properties that facilitate handling and allow attachment to a meniscal tear interface or layering over the repair (Figs. 3 and 4). In addition, PRF in vitro demonstrated favorable elution characteristics compared to PRP in terms of duration and quantity of growth factor [32, 33]. Kemmochi et al. compared 17 patients who underwent meniscal repair with PRF and PRP with 5 control patients. PRF was delivered to the repair site using a customized device. At the latest follow-up, there was a similar improvement in clinical outcome and MRI-assessed healing between the intervention and control groups [34].
Fig. 3.
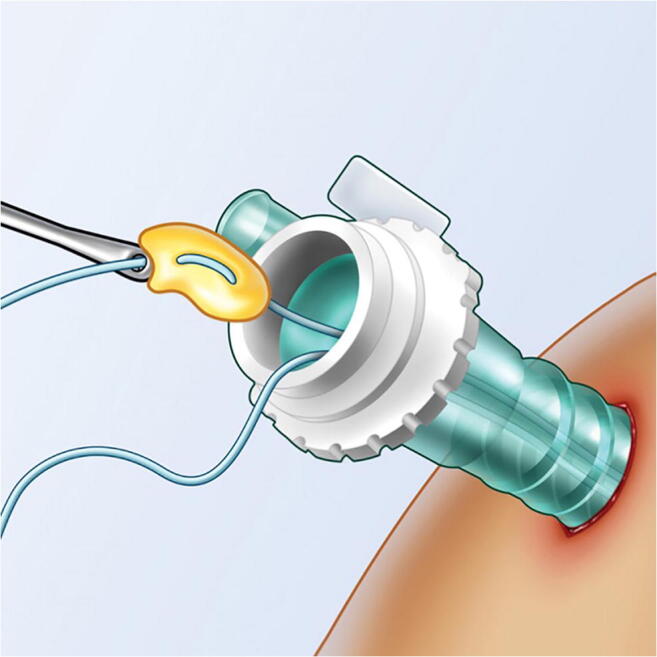
Platelet-rich fibrin is robust enough to be incorporated in the repair construct
Fig. 4.
Platelet-rich fibrin is maintained at the repair site
Ciemniewska-Gorzela et al. reported 5-year patient-reported outcomes and MRI assessment following meniscal repair with a collagen matrix wrapping of each complex tear interface [35•]. Fifty-four patients were treated using the collagen matrix wrapping with bone marrow blood injection; of these, 44 were available for analysis. There was a significant improvement in subjective scores and clinical assessment between the preoperative, 2-year follow-up, and 5-year follow-up time points. Overall survival rate of the repair at final follow-up was 88%. The WORMS osteoarthritis severity grade increased from 6.9 +/− 5.0 points at the 2-year follow-up to 11.1 +/− 9.6 points at the 5-year follow-up, and patients undergoing concomitant ACL reconstruction had significantly more osteoarthritis progression.
Management of the Joint Milieu
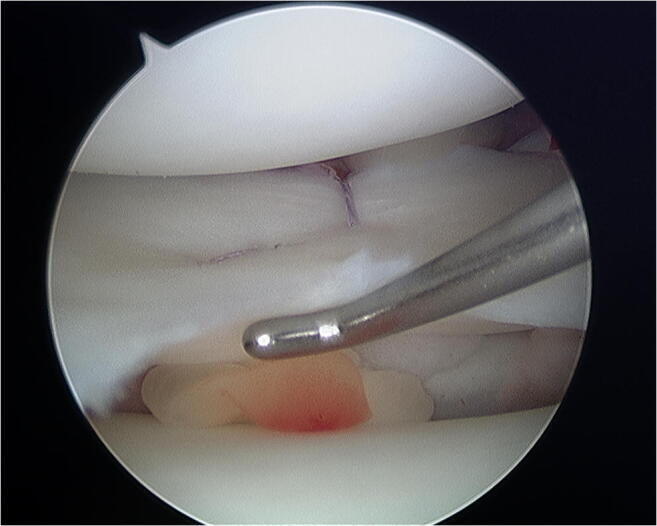
Interest in bone marrow–derived cells and factors to improve meniscal healing was borne out of a positive clinical correlation between ACL reconstruction and meniscal healing rates. The positive association between ACL reconstruction and meniscal repair was observed by Cannon and Vittori who published improved success rates (53 to 93%) compared to isolated meniscus repair [36]. Shelbourne and Heinrich’s long-term follow-up of lateral meniscal tear healing rates similarly demonstrated 96% normal or near-normal results when associated with ACL reconstruction [37]. These findings suggest the potential role of marrow elements in the intra-articular milieu during meniscal healing, notwithstanding the potential confounding role of a slower ACL rehabilitation protocol [38]. With this rationale in mind, the use of microfracture in the intercondylar notch (“marrow venting”) to introduce marrow elements into the knee joint during isolated meniscal repair has been adopted with relative ease of application (Figs. 5 and 6) [39]. Dean et al. reported similar healing rates of isolated meniscal tears augmented with marrow venting to those undertaken in the setting of ACL reconstruction, supporting the role of marrow venting in meniscus repair augmentation [40••].
Fig. 5.
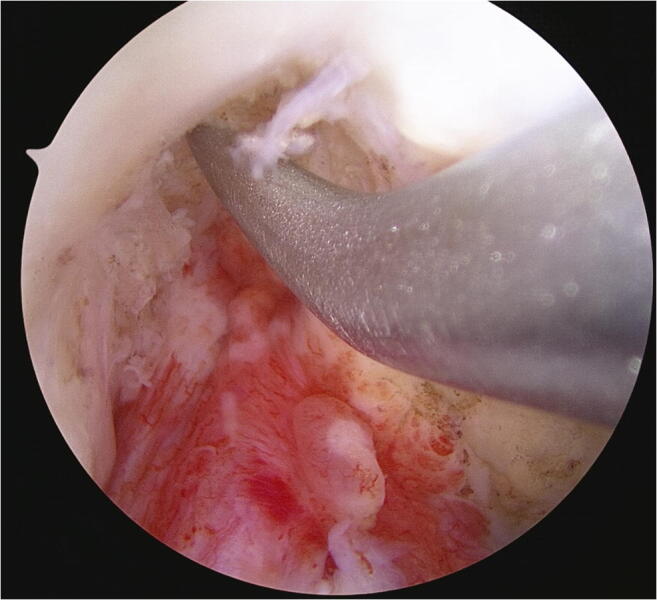
Marrow venting: An awl is used to microfracture the intercondylar notch
Fig. 6.
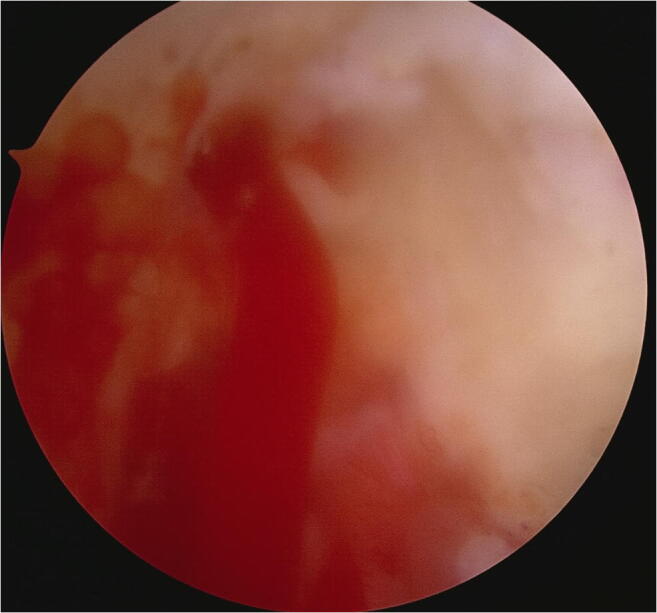
Marrow venting: Marrow elements enter the joint through the microfracture holes
Characterization of the cytokines in bone marrow concentrate (BMC) has confirmed an anti-inflammatory profile that may be advantageous in the setting of meniscal repair [41]. This concept is distinct from the utilization of BMC preparations to provide a source of mesenchymal stromal cells (MSC) for healing as the MSC content is small, accounting for only 0.01–0.02% of the cellular composition of BMC [42]. Koch et al. reported enhanced meniscal healing in vivo for an avascular meniscal tear in a rabbit model treated with BMC when compared to both platelet-rich plasma (PRP) and untreated controls [43].
PRP formulations offer a point of care strategy to deliver various growth factors to healing meniscus tissue. Although there is tremendous variability in clinically available PRP formulations with no single standard definition, some have defined PRP as containing a platelet concentration of at least 1,000,000 platelets/μL in 5 mL of plasma representing a threefold to fivefold increase in concentration over normal circulating platelet numbers. PRP has been shown to contain various growth factors, including transforming growth factor beta-1 (TGF-ß1), fibroblast growth factor-2 (FGF-2), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF), all of which are implicated in the desired biological goals of cell chemotaxis, cell differentiation, angiogenesis, and extracellular matrix production [41]. However, it is well recognized that the variability in techniques for preparation of PRP results in significant heterogeneity in composition, with potential impacts on biological effects [44]. Ishida et al. demonstrated that meniscal cells (fibrochondrocytes) cultured in PRP demonstrated enhanced expression of mRNA for extracellular matrix (ECM) proteins relative to the controls. In vivo, enhanced healing of full-thickness meniscal tears created in the avascular region in a rabbit model was observed following PRP treatment using a gelatin hydrogel delivery system [45].
In the clinical setting, Pujol et al. followed a cohort of 34 patients following open repair of a horizontal meniscal tear: 17 with PRP and 17 without [46]. At 2 years, the PRP group demonstrated better KOOS and sport-related outcomes compared to the control, and complete or partial healing of the meniscal tear was seen more often in the PRP group on follow-up MRI (75% vs 40%). Dai et al. investigated the effect of PRP to augment lateral discoid meniscal tear inside-out repair [47]. Comparing 14 PRP patients to 15 control patients, there was no difference in clinical outcome scores or retear rate. Griffin et al. compared the clinical outcomes of inside-out meniscal repair with PRP (15 patients) and without PRP (20 patients) [48]. They also failed to demonstrate a difference in reoperation rate or clinical outcome measures, although this study had limited power. Everhart et al. reported on a larger cohort of 550 patients investigating the effect of two PRP preparations for isolated meniscal repair and meniscal repair associated with anterior cruciate ligament (ACL) reconstruction [49•]. Their findings at 3 years supported a decreased failure risk (partial meniscectomy/TKA) in the PRP groups compared to the control (14.6% vs 17%) for isolated meniscal tears only; the positive effect of PRP was not observed following concomitant ACL reconstruction. Kaminski et al. conducted a prospective, double-blinded, placebo-controlled randomized controlled trial (RCT) comparing leukocyte-rich PRP with saline control (with trephination for both groups) in 37 patients and reported enhanced meniscal healing rates on MRI/second look arthroscopy (85% vs 47%, P < 0.005) [50••].
Cell-Based Approaches
Cell-based approaches to meniscal repair and regeneration may be comprehensively scrutinized using the Minimum Information for studies evaluating Biologics in Orthopaedics (MIBO) consensus checklist [51••]. Application of autogenous or allogeneic cells represents another promising approach to improving meniscal healing and regeneration. The potential role of exogenous cells is further exemplified by the observation that following acute meniscal injury, synovium-derived MSCs can be found in synovial tissue [52]. The meniscus also contains resident progenitor cells in all three zones that represent a future target for intrinsic tissue cell homing [9].
Zellner et al. investigated the potential of precultured, prochondrocytic MSCs and undifferentiated MSCs on a hyaluronan scaffold in a rabbit model, reporting that undifferentiated MSCs resulted in superior meniscal restoration supporting the role of MSCs as a source of both cells and trophic mediators of repair [53]. Moriguchi et al. demonstrated consistent healing of avascular meniscal tears in a miniature swine model using a tissue-engineered scaffold-free construct derived from synovial MSCs [54]. Ozeki et al. reported enhanced meniscal repair using allogeneic synovial MSCs in a microminipig model at 8 weeks using a comprehensive assessment involving quantitative MRI, polarized light microscopy, and histology [55]. Whitehouse et al. performed a clinical study on patients to investigate the safety of applying MSCs to an avascular meniscal tear as part of a comprehensive translational approach [56••]. Five patients underwent autologous MSC harvest from the iliac crest; these cells were placed in culture for 13 days and underwent immunohistochemical assessment prior to seeding on a collagen scaffold and implantation with suture fixation. At 2 years, 3/5 patients exhibited functional improvement and had no MRI evidence of retear. However, at 15 months, 2/5 patients required a return to the operating room for partial menisectomy due to symptomatic retear [56••]. Seyika et al. enrolled five patients to receive MSCs derived from the synovial tissue of the suprapatellar pouch following repair of a complex, degenerative medial meniscal tear [57••]. After digestion, the cells were cultured in autologous serum for 14 days. The cells were injected at the meniscal repair site 14 days after the initial arthroscopic repair. At 2 years, total Lysholm knee score, the Knee Injury and Osteoarthritis Outcome Scale ( KOOS) scores for “pain,” “daily living,” and “sports activities,” and the Numerical Rating Scale were significantly improved, 3-D MRI demonstrated healing, and there was no revision surgery.
Vangsness et al. conducted a randomized double-blinded controlled study investigating the safety of intra-articular injection of allogeneic MSCs into the knee, performed 7 to 10 days following partial meniscectomy [58]. Longitudinal MRI scans were performed over a 2-year period to observe osteoarthritis progression and to perform volumetric assessment of the operated meniscus. Overall, 55 patients across 7 institutions were randomized to 3 treatment groups: (i) injection of 50 million allogeneic MSCs; (ii) injection of 150 million MSCs, and (iii) a control group receiving the sodium hyaluronate vehicle alone. Meniscal regeneration was measured using an a priori threshold of 15% increase in meniscal volume; 24% of patients who received 50 million cells had a significant increase in meniscal volume compared to 6% of patients in the higher dose group (150 million cells); no volumetric increase was seen in the control group. In patients with established osteoarthritic changes, MSC administration was associated with improvements in Visual Analogue Score (VAS) pain scores across both groups. Importantly, no clinically important safety issues including ectopic tissue formation were encountered.
Rehabilitation, Mechanobiology, and Modalities
General consensus regarding the optimal approach to rehabilitation following meniscal repair with or without biological augmentation remains elusive. Specific tear and procedure-related characteristics dictate progression with obvious concerns for the integrity of the repair, making clear the importance of establishing open communication between the surgeon and physical therapist. In vivo studies have demonstrated that prolonged immobilization of the repaired meniscus results in decreased collagen formation at the repair site [59]. In addition, immobilization of an intact meniscus in the rabbit model resulted in decreased permeability and degenerative changes in the deep layers of the meniscus [60]. At the cellular level, in vitro mechanobiology studies suggest that tensile strain to the meniscal fibrochondrocyte may reduce the inflammatory phenotye and enhance glycosaminoglycan production [61]. Regarding specific modalities, blood flow restriction (BFR) exercise has shown promise as an adjunct in overall lower extremity rehabilitation. Recently, Callanan et al. investigated the impact of performing BRF exercise prior to the procurement of peripheral blood–derived biologics [62••]. Specifically, they investigated the proportions of CD34+ cells, platelets, white blood cells, neutrophils, lymphocytes, lactate, and glucose. In 14 healthy individuals, exercise with BFR caused a significant post-exercise increase in peripheral hematopoietic progenitor cells and platelets compared to standard resistance training, representing a potential opportunity to optimize point of care biological strategies.
In the senior author’s institution, rehabilitation following meniscal repair consists of use of a double upright hinged brace to allow 0–90° flexion for the first 4 weeks. Weight-bearing is graduated in full extension in the brace; weight-bearing without the brace begins at 4–6 weeks post operatively. While partial weight-bearing with the knee in full extension appears safe following repair of a vertical longitudinal tear, a more conservative approach may be considered for repair of a radial tear. Patients are counseled to avoid high-impact athletics for 4–6 months. In athletes, the opportunity to individualize rehabilitation protocols is heavily reliant on open communication between the therapist, athletic trainer, and surgeon with a desired trend towards accelerated, integrated protocols that may facilitate return to sport without compromising career goals of the athlete or their long-term knee joint health.
Repurposing of Medication for Meniscal Injury and Repair
Recent heightened interest in the repurposing of FDA-approved medications for other clinical indications has percolated from other medical specialties into orthopedic research. Repurposed medications are attractive as they offer a potentially easier route to clinical utilization and often have well-established patient safety profiles [63]. Zhang et al. investigated the effect of local hydrogel delivery of simvastatin on meniscal healing in the rabbit meniscal avascular defect model. Their findings supported significantly more repair tissue in the simvastatin group than in the control and this finding was coincident with increased types I and II collagen and BMP 2 and 7 at 12 weeks [64••]. The repair tissue was stiffer, and there was an upregulation in COL1 and COL2. Losartan is an antihypertensive medication with selective angiotensin II type 1 receptor antagonism. It also functions to inhibit TGF-ß1, exerting a dampening effect on the TGF-ß1-Smad pathway and collagen expression. Losartan has been applied in strategies to prevent fibrosis in musculoskeletal tissues [65]. Losartan also exerts effects on endothelial–mesenchymal transition (EndMT), a key element of cytoskeleton remodeling and scar tissue formation following cardiac and musculoskeletal injury. With these mechanisms in mind, Nakama et al. investigated the effect of systemic losartan on point-of-care biologics in the rabbit osteochondral injury and repair model [66]. Their findings supported a partial block of EndMT with increased CD31+ and decreased CD45+ cells (hematopoietic cells) in BMC following losartan administration; there was no effect of oral losartan on TGF-ß1 levels in leukocyte-poor PRP. The same group expanded their investigation in the rabbit osteochondral model and demonstrated superior hyaline-like cartilage repair following microfracture in the rabbits receiving systemic losartan; these findings were co-incident with downregulation of signaling TGF-ß1 in the synovial tissue compared to those rabbits not treated with losartan [67]. Although studies investigating the specific effect of losartan on meniscal healing are lacking, it may hold promise in the future. Finally, management of the pro-inflammatory intraarticular milieu following meniscal injury and repair using repurposed medications is another promising approach. Established disease-modifying arthritis drugs with specific cytokine inhibition have been suggested to optimize the intraarticular environment during healing and recovery. Some cytokine inhibitors that might be considered include anakinra (Kineret®, IL-1ß), entanercept (Enbrel®, TNF-α), and tocilizumab (Actemra®, IL-6). Ultimately, well-designed randomized controlled trials are warranted; the proposed MOCHA trial (ClinicalTrials.gov, NCT04572256) investigating the chondroprotective effects of montelukast (Singulair®, a leukotriene receptor antagonist, used for the treatment of asthma) following ACL injury and reconstruction may provide a comparable precedent [68].
Conclusions
In clinical practice, biological augmentation of meniscal healing and regeneration may be applied to a spectrum of clinical scenarios in an attempt to improve meniscus healing. However, further study is required to carefully characterize the biologic agents used and to objectively measure their effect on meniscus healing and regeneration in order to further our insight into clinical translation of these treatments. Consensus on the nomenclature and reporting of biological treatments (including autogenous and allogeneic cell sources) represents a step forward for orthobiologics. Finally, local meniscal and synovial tissue stimulation, tear interfacial treatments, and management of the intraarticular milieu represent a comprehensive approach to optimizing the biological aspects of meniscus healing.
Acknowledgements
The authors would like to acknowledge
Declarations
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Meniscus
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411–7431. doi: 10.1016/j.biomaterials.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutchinson ID, Rodeo SA, Perrone GS, Murray MM. Can platelet-rich plasma enhance anterior cruciate ligament and meniscal repair? The journal of knee surgery. 2014. [DOI] [PubMed]
- 3.McDermott I. Meniscal tears, repairs and replacement: their relevance to osteoarthritis of the knee. Br J Sports Med. 2011;45(4):292–297. doi: 10.1136/bjsm.2010.081257. [DOI] [PubMed] [Google Scholar]
- 4.Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis and rheumatism. 1998;41(4):687–693. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Verdonk R, Verdonk P, Huysse W, Forsyth R, Heinrichs EL. Tissue ingrowth after implantation of a novel, biodegradable polyurethane scaffold for treatment of partial meniscal lesions. The American journal of sports medicine. 2011;39(4):774–782. doi: 10.1177/0363546511398040. [DOI] [PubMed] [Google Scholar]
- 6.•.Zaffagnini S, Poggi A, Reale D, Andriolo L, Flanigan DC, Filardo G. Biologic augmentation reduces the failure rate of meniscal repair: a systematic review and meta-analysis. Orthop J Sports Med. 2021;9(2):2325967120981627. doi: 10.1177/2325967120981627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noyes FR, Barber-Westin SD. Management of meniscus tears that extend into the avascular region. Clin Sports Med. 2012;31(1):65–90. doi: 10.1016/j.csm.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Maak TG, Fabricant PD, Wickiewicz TL. Indications for meniscus repair. Clin Sports Med. 2012;31(1):1–14. doi: 10.1016/j.csm.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 9.••.Chahla J, Papalamprou A, Chan V, Arabi Y, Salehi K, Nelson TJ, et al. Assessing the resident progenitor cell population and the vascularity of the adult human meniscus. Arthroscopy. 2021;37(1):252–265. doi: 10.1016/j.arthro.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnoczky SP, Warren RF. Microvasculature of the human meniscus. The American journal of sports medicine. 1982;10(2):90–95. doi: 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- 11.Grant JA, Wilde J, Miller BS, Bedi A. Comparison of inside-out and all-inside techniques for the repair of isolated meniscal tears: a systematic review. The American journal of sports medicine. 2012;40(2):459–468. doi: 10.1177/0363546511411701. [DOI] [PubMed] [Google Scholar]
- 12.Rodeo SA, Bedi A. 2019-2020 NFL and NFL Physician Society Orthobiologics Consensus Statement. Sports Health. 2020;12(1):58–60. doi: 10.1177/1941738119889013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narita A, Takahara M, Sato D, Ogino T, Fukushima S, Kimura Y, et al. Biodegradable gelatin hydrogels incorporating fibroblast growth factor 2 promote healing of horizontal tears in rabbit meniscus. Arthroscopy. 2012;28(2):255–263. doi: 10.1016/j.arthro.2011.08.294. [DOI] [PubMed] [Google Scholar]
- 14.He W, Liu YJ, Wang ZG, Guo ZK, Wang MX, Wang N. Enhancement of meniscal repair in the avascular zone using connective tissue growth factor in a rabbit model. Chin Med J (Engl). 2011;124(23):3968–3975. [PubMed] [Google Scholar]
- 15.Kopf S, Birkenfeld F, Becker R, Petersen W, Starke C, Wruck CJ, et al. Local treatment of meniscal lesions with vascular endothelial growth factor. The Journal of bone and joint surgery American volume. 2010;92(16):2682–2691. doi: 10.2106/JBJS.I.01481. [DOI] [PubMed] [Google Scholar]
- 16.Hutchinson ID, Moran CJ, Potter HG, Warren RF, Rodeo SA. Restoration of the meniscus: form and function. Am J Sports Med. 2014;42(4):987–998. doi: 10.1177/0363546513498503. [DOI] [PubMed] [Google Scholar]
- 17.Ochi M, Uchio Y, Okuda K, Shu N, Yamaguchi H, Sakai Y. Expression of cytokines after meniscal rasping to promote meniscal healing. Arthroscopy. 2001;17(7):724–731. doi: 10.1053/jars.2001.23583. [DOI] [PubMed] [Google Scholar]
- 18.Arnoczky SP, Warren RF. The microvasculature of the meniscus and its response to injury. An experimental study in the dog. The American journal of sports medicine. 1983;11(3):131–141. doi: 10.1177/036354658301100305. [DOI] [PubMed] [Google Scholar]
- 19.Nakhostine M, Gershuni DH, Danzig LA. Effects of an in-substance conduit with injection of a blood clot on tears in the avascular region of the meniscus. Acta Orthop Belg. 1991;57(3):242–246. [PubMed] [Google Scholar]
- 20.Fox JM, Rintz KG, Ferkel RD. Trephination of incomplete meniscal tears. Arthroscopy. 1993;9(4):451–455. doi: 10.1016/S0749-8063(05)80321-4. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Arnold JA. Trephination and suturing of avascular meniscal tears: a clinical study of the trephination procedure. Arthroscopy. 1996;12(6):726–731. doi: 10.1016/S0749-8063(96)90178-4. [DOI] [PubMed] [Google Scholar]
- 22.Arnoczky SP, Warren RF, Spivak JM. Meniscal repair using an exogenous fibrin clot. An experimental study in dogs. The Journal of bone and joint surgery American volume. 1988;70(8):1209–1217. doi: 10.2106/00004623-198870080-00012. [DOI] [PubMed] [Google Scholar]
- 23.Kamimura T, Kimura M. Repair of horizontal meniscal cleavage tears with exogenous fibrin clots. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1154–1157. doi: 10.1007/s00167-011-1404-5. [DOI] [PubMed] [Google Scholar]
- 24.Shoji T, Nakasa T, Yoshizuka M, Yamasaki T, Yasunaga Y, Adachi N, et al. Comparison of fibrin clots derived from peripheral blood and bone marrow. Connect Tissue Res. 2017;58(2):208–214. doi: 10.1080/03008207.2016.1215443. [DOI] [PubMed] [Google Scholar]
- 25.Henning CE, Lynch MA, Yearout KM, Vequist SW, Stallbaumer RJ, Decker KA. Arthroscopic meniscal repair using an exogenous fibrin clot. Clin Orthop Relat Res. 1990;252:64–72. doi: 10.1097/00003086-199003000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Henning CE, Yearout KM, Vequist SW, Stallbaumer RJ, Decker KA. Use of the fascia sheath coverage and exogenous fibrin clot in the treatment of complex meniscal tears. Am J Sports Med. 1991;19(6):626–631. doi: 10.1177/036354659101900613. [DOI] [PubMed] [Google Scholar]
- 27.Yamanashi Y, Kato T, Akao M, Takata T, Kobayakawa K, Deie M. Meniscal repair using fibrin clots made from bone marrow blood wrapped in a polyglycolic acid sheet. Arthrosc Tech. 2021;10(11):e2541–e25e6. doi: 10.1016/j.eats.2021.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Trommel MF, Simonian PT, Potter HG, Wickiewicz TL. Arthroscopic meniscal repair with fibrin clot of complete radial tears of the lateral meniscus in the avascular zone. Arthroscopy. 1998;14(4):360–365. doi: 10.1016/S0749-8063(98)70002-7. [DOI] [PubMed] [Google Scholar]
- 29.Ra HJ, Ha JK, Jang SH, Lee DW, Kim JG. Arthroscopic inside-out repair of complete radial tears of the meniscus with a fibrin clot. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2126–2130. doi: 10.1007/s00167-012-2191-3. [DOI] [PubMed] [Google Scholar]
- 30.Jang SH, Ha JK, Lee DW, Kim JG. Fibrin clot delivery system for meniscal repair. Knee surgery & related research. 2011;23(3):180–183. doi: 10.5792/ksrr.2011.23.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamimura T, Kimura M. Meniscal repair of degenerative horizontal cleavage tears using fibrin clots: clinical and arthroscopic outcomes in 10 cases. Orthop J Sports Med. 2014;2(11):2325967114555678. doi: 10.1177/2325967114555678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutchinson ID, Rodeo SA, Perrone GS, Murray MM. Can platelet-rich plasma enhance anterior cruciate ligament and meniscal repair? J Knee Surg. 2015;28(1):19–28. doi: 10.1055/s-0034-1387166. [DOI] [PubMed] [Google Scholar]
- 33.Schar MO, Diaz-Romero J, Kohl S, Zumstein MA, Nesic D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin Orthop Relat Res. 2015;473(5):1635–1643. doi: 10.1007/s11999-015-4192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kemmochi M, Sasaki S, Takahashi M, Nishimura T, Aizawa C, Kikuchi J. The use of platelet-rich fibrin with platelet-rich plasma support meniscal repair surgery. J Orthop. 2018;15(2):711–720. doi: 10.1016/j.jor.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.•.Ciemniewska-Gorzela K, Bakowski P, Naczk J, Jakob R, Piontek T. Complex meniscus tears treated with collagen matrix wrapping and bone marrow blood injection: clinical effectiveness and survivorship after a minimum of 5 years' follow-up. Cartilage. 2021;13(1_suppl):228S–238S. doi: 10.1177/1947603520924762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cannon WD, Jr, Vittori JM. The incidence of healing in arthroscopic meniscal repairs in anterior cruciate ligament-reconstructed knees versus stable knees. Am J Sports Med. 1992;20(2):176–181. doi: 10.1177/036354659202000214. [DOI] [PubMed] [Google Scholar]
- 37.Shelbourne KD, Heinrich J. The long-term evaluation of lateral meniscus tears left in situ at the time of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20(4):346–351. doi: 10.1016/j.arthro.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 38.Hutchinson ID, Moran CJ, Potter HG, Warren RF, Rodeo SA. Restoration of the Meniscus: form and function. The American journal of sports medicine. 2013. [DOI] [PubMed]
- 39.Freedman KB, Nho SJ, Cole BJ. Marrow stimulating technique to augment meniscus repair. Arthroscopy. 2003;19(7):794–798. doi: 10.1016/S0749-8063(03)00695-9. [DOI] [PubMed] [Google Scholar]
- 40.••.Dean CS, Chahla J, Matheny LM, Mitchell JJ, RF LP. Outcomes after biologically augmented isolated meniscal repair with marrow venting are comparable with those after meniscal repair with concomitant anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(6):1341–1348. doi: 10.1177/0363546516686968. [DOI] [PubMed] [Google Scholar]
- 41.Ziegler CG, Van Sloun R, Gonzalez S, Whitney KE, DePhillipo NN, Kennedy MI, et al. Characterization of growth factors, cytokines, and chemokines in bone marrow concentrate and platelet-rich plasma: a prospective analysis. Am J Sports Med. 2019;47(9):2174–2187. doi: 10.1177/0363546519832003. [DOI] [PubMed] [Google Scholar]
- 42.Schafer R, DeBaun MR, Fleck E, Centeno CJ, Kraft D, Leibacher J, et al. Quantitation of progenitor cell populations and growth factors after bone marrow aspirate concentration. J Transl Med. 2019;17(1):115. doi: 10.1186/s12967-019-1866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koch M, Hammer S, Fuellerer J, Lang S, Pfeifer CG, Pattappa G, et al. Bone marrow aspirate concentrate for the treatment of avascular meniscus tears in a one-step procedure-evaluation of an in vivo model. Int J Mol Sci. 2019;20(5). [DOI] [PMC free article] [PubMed]
- 44.Oudelaar BW, Peerbooms JC, In H, 't Veld R, Vochteloo AJH. Concentrations of blood components in commercial platelet-rich plasma separation systems: a review of the literature. Am J Sports Med. 2019;47(2):479–87. [DOI] [PubMed]
- 45.Ishida K, Kuroda R, Miwa M, Tabata Y, Hokugo A, Kawamoto T, et al. The regenerative effects of platelet-rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tissue engineering. 2007;13(5):1103–1112. doi: 10.1089/ten.2006.0193. [DOI] [PubMed] [Google Scholar]
- 46.Pujol N, Salle De Chou E, Boisrenoult P, Beaufils P. Platelet-rich plasma for open meniscal repair in young patients: any benefit? Knee Surg Sports Traumatol Arthrosc. 2015;23(1):51–58. doi: 10.1007/s00167-014-3417-3. [DOI] [PubMed] [Google Scholar]
- 47.Dai WL, Zhang H, Lin ZM, Shi ZJ, Wang J. Efficacy of platelet-rich plasma in arthroscopic repair for discoid lateral meniscus tears. BMC Musculoskelet Disord. 2019;20(1):113. doi: 10.1186/s12891-019-2500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffin JW, Hadeed MM, Werner BC, Diduch DR, Carson EW, Miller MD. Platelet-rich plasma in meniscal repair: does augmentation improve surgical outcomes? Clin Orthop Relat Res. 2015;473(5):1665–1672. doi: 10.1007/s11999-015-4170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.•.Everhart JS, Cavendish PA, Eikenberry A, Magnussen RA, Kaeding CC, Flanigan DC. Platelet-rich plasma reduces failure risk for isolated meniscal repairs but provides no benefit for meniscal repairs with anterior cruciate ligament reconstruction. Am J Sports Med. 2019;47(8):1789–96 PRP was more efficacious in the treatment of isolated meniscal tears compared to meniscal tears with concomitant ACL reconstruction. [DOI] [PubMed]
- 50.••.Kaminski R, Kulinski K, Kozar-Kaminska K, Wielgus M, Langner M, Wasko MK, et al. A prospective, randomized, double-blind, parallel-group, placebo-controlled study evaluating meniscal healing, clinical outcomes, and safety in patients undergoing meniscal repair of unstable, complete vertical meniscal tears (bucket handle) augmented with platelet-rich plasma. Biomed Res Int. 2018;2018:9315815. doi: 10.1155/2018/9315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.••.Murray IR, Geeslin AG, Goudie EB, Petrigliano FA, LaPrade RF. Minimum Information for Studies Evaluating Biologics in Orthopaedics (MIBO): platelet-rich plasma and mesenchymal stem cells. J Bone Joint Surg Am. 2017;99(10):809–819. doi: 10.2106/JBJS.16.00793. [DOI] [PubMed] [Google Scholar]
- 52.Matsukura Y, Muneta T, Tsuji K, Koga H, Sekiya I. Mesenchymal stem cells in synovial fluid increase after meniscus injury. Clin Orthop Relat Res. 2014;472(5):1357–1364. doi: 10.1007/s11999-013-3418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zellner J, Mueller M, Berner A, Dienstknecht T, Kujat R, Nerlich M, et al. Role of mesenchymal stem cells in tissue engineering of meniscus. J Biomed Mater Res A. 2010;94(4):1150–1161. doi: 10.1002/jbm.a.32796. [DOI] [PubMed] [Google Scholar]
- 54.Moriguchi Y, Tateishi K, Ando W, Shimomura K, Yonetani Y, Tanaka Y, et al. Repair of meniscal lesions using a scaffold-free tissue-engineered construct derived from allogenic synovial MSCs in a miniature swine model. Biomaterials. 2013;34(9):2185–2193. doi: 10.1016/j.biomaterials.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 55.Ozeki N, Koga H, Sekiya I. Homeostasis and disorder of musculoskeletal system. Transplantation of synovial mesenchymal stem cells for cartilage and meniscus regeneration. Clin Calcium. 2018;28(3):319–327. [PubMed] [Google Scholar]
- 56.••.Whitehouse MR, Howells NR, Parry MC, Austin E, Kafienah W, Brady K, et al. Repair of torn avascular meniscal cartilage using undifferentiated autologous mesenchymal stem cells: from in vitro optimization to a first-in-human study. Stem Cells Transl Med. 2017;6(4):1237-48. Clinical study of the use of expanded autogenous bone marrow–derived progenitor cells seeded on a collagen scaffold and applied to meniscal tears with 3/5 (60%) positive outcome at 2 years. [DOI] [PMC free article] [PubMed]
- 57.••.Sekiya I, Koga H, Otabe K, Nakagawa Y, Katano H, Ozeki N, et al. Additional use of synovial mesenchymal stem cell transplantation following surgical repair of a complex degenerative tear of the medial meniscus of the knee: a case report. Cell Transplant. 2019;28(11):1445–1454. doi: 10.1177/0963689719863793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vangsness CT, Jr, Farr J, 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96(2):90–98. doi: 10.2106/JBJS.M.00058. [DOI] [PubMed] [Google Scholar]
- 59.Dowdy PA, Miniaci A, Arnoczky SP, Fowler PJ, Boughner DR. The effect of cast immobilization on meniscal healing. An experimental study in the dog. Am J Sports Med. 1995;23(6):721–728. doi: 10.1177/036354659502300615. [DOI] [PubMed] [Google Scholar]
- 60.Ochi M, Kanda T, Sumen Y, Ikuta Y. Changes in the permeability and histologic findings of rabbit menisci after immobilization. Clin Orthop Relat Res. 1997;334:305–315. doi: 10.1097/00003086-199701000-00040. [DOI] [PubMed] [Google Scholar]
- 61.McNulty AL, Guilak F. Mechanobiology of the meniscus. J Biomech. 2015;48(8):1469–1478. doi: 10.1016/j.jbiomech.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.•.Callanan MC, Plummer HA, Chapman GL, Opitz TJ, Rendos NK, Anz AW. Blood flow restriction training using the delfi system is associated with a cellular systemic response. Arthrosc Sports Med Rehabil. 2021;3(1):e189–ee98. doi: 10.1016/j.asmr.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hutchinson ID, Ata A, DiCaprio MR. Is metformin use associated with prolonged overall survival in patients with soft tissue sarcoma? A SEER-Medicare Study. Clin Orthop Relat Res. 2022;480(4):735–744. doi: 10.1097/CORR.0000000000002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.•.Zhang S, Matsushita T, Kuroda R, Nishida K, Matsuzaki T, Matsumoto T, et al. Local administration of simvastatin stimulates healing of an avascular meniscus in a rabbit model of a meniscal defect. Am J Sports Med. 2016;44(7):1735–1743. doi: 10.1177/0363546516638342. [DOI] [PubMed] [Google Scholar]
- 65.Huard J, Bolia I, Briggs K, Utsunomiya H, Lowe WR, Philippon MJ. Potential usefulness of losartan as an antifibrotic agent and adjunct to platelet-rich plasma therapy to improve muscle healing and cartilage repair and prevent adhesion formation. Orthopedics. 2018;41(5):e591–e5e7. doi: 10.3928/01477447-20180806-05. [DOI] [PubMed] [Google Scholar]
- 66.Nakama GY, Gonzalez S, Matre P, Mu X, Whitney KE, Utsunomiya H, et al. Effect of oral losartan on orthobiologics: implications for platelet-rich plasma and bone marrow concentrate-a rabbit study. Int J Mol Sci. 2020;21(19). [DOI] [PMC free article] [PubMed]
- 67.Utsunomiya H, Gao X, Deng Z, Cheng H, Nakama G, Scibetta AC, et al. Biologically regulated marrow stimulation by blocking TGF-beta1 with losartan oral administration results in hyaline-like cartilage repair: a rabbit osteochondral defect model. Am J Sports Med. 2020;48(4):974–984. doi: 10.1177/0363546519898681. [DOI] [PubMed] [Google Scholar]
- 68.Jacobs CA, Conley CEW, Kraus VB, Lansdown DA, Lau BC, Li X, et al. MOntelukast as a potential chondroprotective treatment following anterior cruciate ligament reconstruction (MOCHA Trial): study protocol for a double-blind, randomized, placebo-controlled clinical trial. Trials. 2022;23(1):98. doi: 10.1186/s13063-021-05982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


