Figure 13.
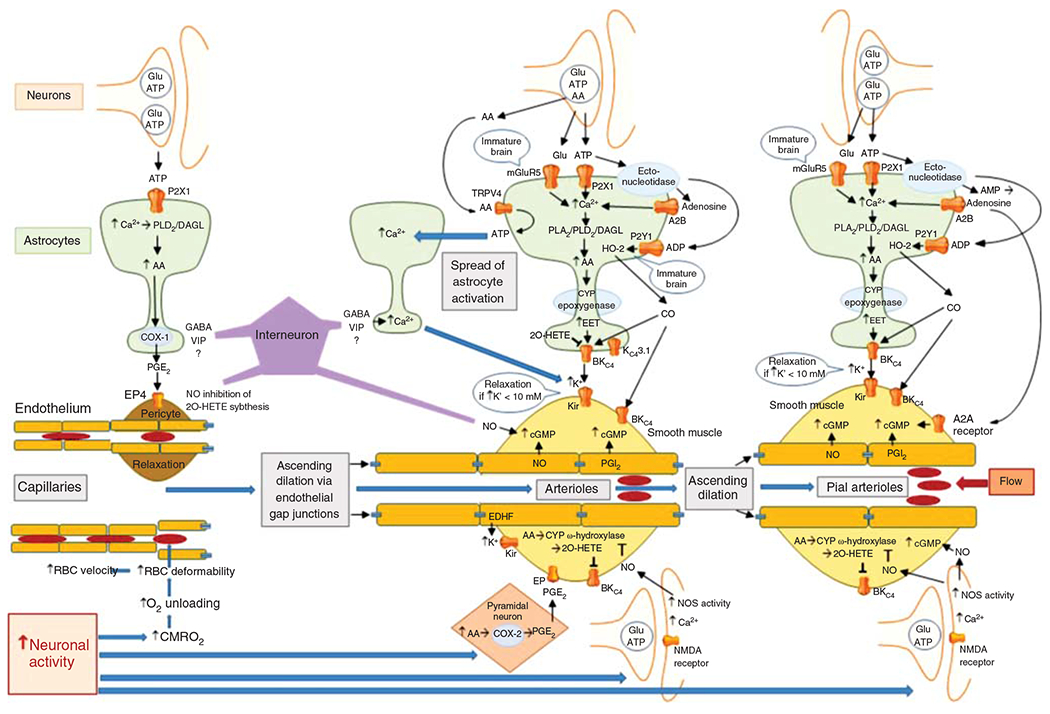
Schematic diagram of the major signaling pathways involved in neurovascular coupling. With the initiation of neuronal action, the sequence of events proceeds from left to right, resulting in ascending vasodilation, part of which depends on conduction through endothelial gap junctions. The first step occurs with the increase in CMRO2 leading to a transient increase in deoxyhemoglobin that results in an increase in red blood cell (RBC) deformability and velocity in capillaries. The second step involves release of the coneurotransmitter ATP acting on astrocyte P2X1 receptors to stimulate mobilization of arachidonic acid (AA) via phospholipase D2 (PLD2) and diacylglycerol lipase (DGL). PGE2 is synthetized by cyclooxygenase-1 (COX-1) and released from the astrocyte foot process to induce relaxation of pericytes via EP4 receptors. Relaxation of circumferential pericytes increases capillary diameter in the vicinity of terminal arterioles, thereby increasing RBC flux. In addition to ATP, some interneurons in close proximity to astrocyte foot processes release GABA and VIP that either directly or indirectly act to increase Ca2+ in the foot processes adjacent to pericytes and smooth muscle and that act to facilitate rapid vasorelaxation signaling. The third step is the action of neurotransmitters on astrocytes adjacent to vascular smooth muscle of intraparenchymal arterioles. In addition to the effects of ATP on astrocyte P2X1 receptors, glutamate can act on metabotropic glutamate receptor 5 (mGluR5) that is thought to be present on astrocytes of immature brain. Extracellular ATP can be metabolized by ectonucleotidases to adenosine, which in sufficiently high concentration can activate the low-affinity adenosine A2B receptor. Together, these pathways may facilitate Ca2+ wave transmission throughout the astrocyte network leading to mobilization of AA, which can act as a substrate for cytochrome P450 (CYP) epoxygenase to produce epoxyeicosatrienoic acids (EETs). EETs can activate BKCa channels on the astrocyte foot process and release sufficient K+ into the tight extracellular space between the foot process and smooth muscle to activate Kir channels on smooth muscle, resulting in hyperpolarization and relaxation. Furthermore, ATP can be metabolized by a different ectonucleotidase on astrocytes to ADP, which can lead to activation of heme oxygenase-2 (HO-2) expressed in astrocytes of immature brain. HO-2 generates CO, which in swine can facilitate the opening of BKCa channels on smooth muscle and possibly on astrocytes. The intermediate conductance KCa channel 3.1 and the TRPV4 channel on astrocytes also play a less well-defined role in the coupling process. AA can be released as a coneurotransmitter and serve as an agonist on TRPV4 channels that signal the release of ATP into the extracellular space where they can act on adjacent astrocyte processes to produce a Ca2+ response. This slow spread of Ca2+ waves in surrounding astrocytes presumably augments vasorelaxation signaling converging on smooth muscle. In addition to astrocyte-mediated vasodilation, neurons can exert direct effects on smooth muscle, including release of PGE2 generated by COX-2 in select neurons and release of NO by neuronal NO synthase (NOS) mediated by NMDA receptors. The NO can stimulate guanylyl cyclase and increase vasorelaxant cGMP in smooth muscle. NO can also inhibit CYP ω-hydroxylase activity and the generation of 20-HETE that normally inhibits BKCa channels. Some GABAergic interneurons have processes in close proximity to arterioles and capillaries and are thought to induce relaxation via an NO-dependent mechanism. The fourth step is dilation of pial arterioles to maintain the perfusion pressure for the penetrating arterioles serving the activated and nonactivated regions. This dilation involves some of the same mechanisms mediating penetrating arteriolar dilation, including NO generated by neuronal NOS and CO generated by HO-2 in astrocytes in the glia limitans. One difference is that the astrocyte-derived adenosine acts directly on pial arteriole smooth muscle A2A receptors to produce dilation.
