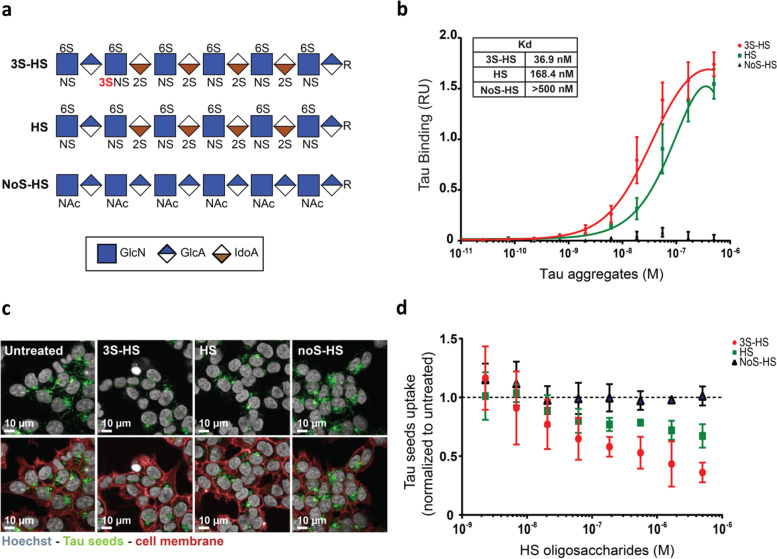
Fig. 3.
Tau PFFs bind with higher affinity to 3S-HS, resulting in a decreased uptake. a Structure and sulfation pattern of the synthetic heparan sulfates: 3-O sulfated (3S-HS), non-3-O sulfated (HS) and non-sulfated (NoS-HS) oligosaccharides. GlcN: glucosamine, GlcA: glucuronic acid, IdoA: iduronic acid. b Using synthetic oligosaccharides immobilized on an ELISA plate, followed by incubation with tau PFFs in a dose-response, it is possible to observe a higher affinity of tau PFFs to the 3S-HS structure (Kd = 36.9 nM), comparing to the HS structure (Kd = 168.4 nM). As expected, tau PFFs showed no binding to the NoS-HS structure (Kd > 500 nM). Experiment was run in triplicates and data is represented as mean ± SD (c) Using live-cell microscopy to measure tau-AF488 uptake in HCT-116 WT cells, a reduction on the number and intensity of tau-AF488 seeds puncta can be observed after pre-treatment with either 3S-HS or HS oligosaccharides. Yet the observed decrease in tau uptake is larger after 3S-HS treatment, when compared to targeting with HS. NoS-HS treatment shows no effect on the number and intensity of tau-AF488 puncta. d Quantification of tau-AF488 seeds uptake by HCT-116 WT cells shows a dose-dependent increase in inhibition of tau uptake by the sulfated oligosaccharides, with 3S-HS oligosaccharide demonstrating an inhibition of 65% at the highest dose on tau seeds uptake. Data represented as mean ± SD of 3 independent experiments