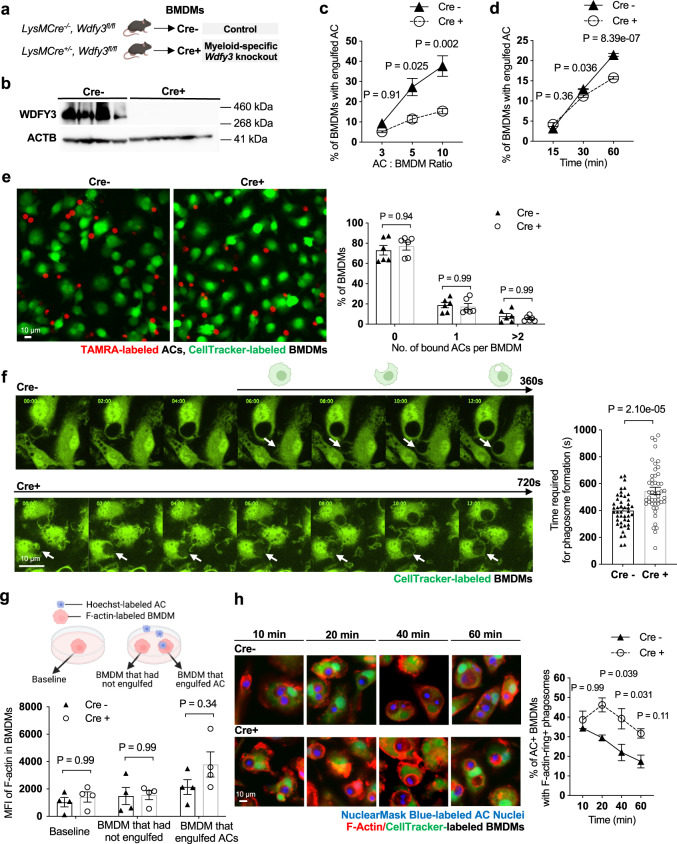
Fig. 2. WDFY3 deficiency led to impaired uptake, as opposed to binding, of apoptotic cells (ACs) due to defective actin depolymerization.
a Schematics of breeding LysMCre mice with Wdfy3fl/fl mice to obtain mice with myeloid-specific knockout of Wdfy3. b Validation of efficient knockout in BMDMs by western blot of WDFY3 (n = 4 biological replicates; the blot shown is a representative image of three independent experiments). c Cre- and Cre+ BMDMs were incubated with Hoechst-labeled ACs at various AC: BMDM ratios of 3:1, 5:1, 10:1 respectively for 1 h and analyzed by flow cytometry (n = 3 biological replicates, each from the average of 2 technical replicates). d Cre- and Cre+ BMDMs were incubated with PKH26-labeled ACs at various time points of 15 min, 30 min, and 60 min at a AC: BMDM ratio of 5:1 and analyzed by flow cytometry (n = 3 technical replicates). e Cre- and Cre+ BMDMs were pretreated with cytochalasin D for 30 min to block polymerization and elongation of actin, thus testing the binding of ACs with BMDMs. The treated BMDMs were then incubated with TAMRA-stained apoptotic mouse thymocytes at 37 °C for 30 min and then extensively washed with DPBS to remove unbound ACs for imaging and quantification after fixation (n = 6 biological replicates). f Cre- and Cre+ BMDMs were stained with CellTracker and incubated with ACs. Efferocytosis of ACs by BMDMs were observed using time-lapse confocal microscopy. The time required for phagosome formation was recorded and quantified (n = 44 and 47 data points for Cre- and Cre+ respectively, each data point represents one BMDM with engulfed ACs, 4 biological replicates for each genotype). The arrows point to the BMDM engulfing an AC across the stages from phagocytic cup formation to phagosome closure (from left to right). g F-actin labeled by siR-actin in Cre- and Cre+ BMDMs was quantified by flow cytometry (n = 4 biological replicates, each from the average of 3 technical replicates). h BMDMs were stained with CellTracker and siR-actin, then incubated with NuclearMask Blue-labeled apoptotic Jurkat cells for various time points (10 min, 20 min, 40 min, and 60 min). For each time point, unbound ACs were removed and BMDMs were fixed. BMDMs were imaged and the percentage of BMDMs with engulfed cargos surrounded by F-actin rings in all BMDMs with engulfed cargos was quantified (n = 4 biological replicates, data are representative of two independent experiments). Data are presented as mean ± SEM. Two-sided P values were determined by a two-way ANOVA with Tukey’s multiple comparisons test in (c, d, e, g, h), or by unpaired t test in panel f.