Abstract
Purpose
Chemoresistance is a major factor contributing to the failure of cancer treatment. The conventional chemotherapy agent 5-fluorouracil (5-FU) has been used for cancer treatment for decades. However, its use is limited in the treatment of hepatocellular carcinoma (HCC) due to acquired resistance. Nrf2 (NF-E2-related factor 2) is known to be associated with drug resistance across a wide range of cancer types. Also, since arsenic trioxide (As2O3) showed antitumor effects on HCC, the purpose of this study was to determine whether As2O3 and Nrf2-siRNA could inhibit HCC synergistically.
Methods
We generated two separate 5-FU-resistant HCC cell lines (SNU-387/5-FU and Hep3B/5-FU). Western blotting was used to determine protein levels. An efficient lentiviral delivery system was used to establish stable knockdown or overexpression of Nrf2 and HIF-1α. In vitro and in vivo analyses of the effects of Nrf2 gene knockdown and As2O3 on 5-FU-resistant HCC cells were conducted.
Results
The expression of Nrf2 was higher in the 5-FU-resistant HCC cell lines than in the parental cell lines. When coupled with Nrf2 knockdown, As2O3 treatment significantly decreased 5-FU-resistant SNU-387 and Hep3B cell viability, migration, and invasion, inactivated HIF-1α/HSP70 signaling, inhibited anti-apoptotic B-cell lymphoma (Bcl-2) activity, and increased the expression of pro-apoptotic Bcl-2-associated X protein (BAX) along with caspase-3. The synergistic effect was also confirmed using a 5-FU-resistant Hep3B mouse xenograft model in vivo.
Conclusion
Nrf2 knockdown could improve the effect of As2O3 on reversing drug resistance in 5-FU-resistant HCC cells.
Keywords: arsenic trioxide, 5-fluorouracil, chemoresistance, hepatocellular carcinoma, Nrf2, HIF-1α
Introduction
Generally, chemotherapy resistance is associated with tumors containing cancer stem cells, a subpopulation of cells associated with undifferentiated tumors.1 Chemotherapy resistance involves complex processes of cell death avoidance, DNA repair, and changes in drug metabolism.2–4 Among the liver cancer risk factors, oxidative stress causes multidrug resistance.5,6 Only patients with early hepatocellular carcinoma (HCC) can be treated with radical surgery, although this treatment has a poor long-term prognosis due to a high recurrence rate. Systemic therapy plays an increasingly important role in the treatment of HCC due to the complexity of the disease and several newly discovered therapeutic approaches.7,8 Therefore, there is an urgent need to explore alternative treatments to improve the outcome. Although 5-fluorouracil (5-FU) is the preferred treatment for advanced HCC, it is associated with inherent and acquired drug resistance. Despite this, recent clinical trials suggest the combination of 5-FU with other drugs for the treatment of HCC is both safe and effective.9–11 So, combination therapy using traditional chemotherapeutic agents or small molecule inhibitors selectively targeting tumor cells is a promising approach.
HCC is characterized by excessive cell proliferation, which leads to local tissue hypoxia in the process of rapid growth, resulting in redox imbalance in the tumor cells. Hypoxia is one of the environmental factors that lead to chemical resistance. Hypoxia can alter the characteristics of tumor cells, leading to a certain degree of resistance to several chemotherapeutic drugs. Inducing endogenous antioxidant responses via nuclear factor erythroid 2-related factor (Nrf2) is important for regulating oxidative stress response.12–14 Nrf2 plays an important role in resisting oxidative damage triggered by foreign chemicals and maintaining normal redox homeostasis in cells. And recent studies have confirmed that the loss of Nrf2 expression is an important anti-hypoxia mechanism.15,16
Although the activation of Nrf2 may reduce cancer risk by inhibiting oxidative stress and promoting tumor inflammation, it is also associated with a poor prognosis. Arsenic trioxide (As2O3) is the main active ingredient in indigo and arsenic. Our previous studies have confirmed that As2O3 inhibits tumor growth, angiogenesis, and metastasis and has an anti-tumor effect on HCC.17–19 As2O3 induces apoptosis by generating reactive oxygen species, destroying mitochondrial transmembrane potential, downregulating Bcl-2, and activating caspase-3. In addition, reactive oxygen species are known to induce immunogenic tumor cell death. Hypoxia-inducible factor-1α (HIF-1α) is widely expressed in tumor tissues and plays an important role in maintaining tumor cell energy metabolism, tumor angiogenesis, and promoting tumor cell proliferation and metastasis.20 So far, whether the anti-tumor effect of As2O3 and Nrf2 is realized by inhibiting the HIF-1α pathway remains unclear. Therefore, the current study investigated the synergistic interaction between Nrf2 and As2O3 to improve the sensitivity of HCC cells to multiple chemotherapeutic drugs.
Materials and Methods
Cell Culture
The poorly differentiated cell lines SNU-387, SNU-449 and well differentiated cell lines Huh7 and Hep3B were purchased from the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and a 1% antibiotic mixture of penicillin and streptomycin (HyClone, Logan, UT, USA). Cultures were routinely passaged every 2–3 days in a humidified incubator at 37 °C containing 5% CO2. To establish resistant cell lines, SNU-387 and Hep3B cells were re-plated in 100 mm dishes and incubated with increasing 5-FU concentrations from 0.5 μM to 150 μM for approximately 4 months.21 The 5-FU-resistant strains (SNU-387/5-FU and Hep3B/5-FU) were maintained in DMEM containing 10 μM 5-FU.
Lentiviral Transfection
The lentiviral siRNAs against Nrf2 (siNrf2) and HIF-1α (siHIF-1α) and containing Nrf2 protein (pHBLV-GFP-Puro-Nrf2, OE-Nrf2), siRNA-scramble (siRNA), and control lentiviruses (pHBLV-GFP-Puro, OE-vector) were purchased from HanBio Biotechnology, Co., Ltd (Shanghai, China). After determining the optimal multiplicity of infection by performing pre-transfection experiments, the lentiviruses were transfected into the 5-FU-resistant HCC cells (SNU-387/5-FU and Hep3B/5-FU) and the cells were incubated for 72 h. The transfection efficiency was measured using Western blotting.
Cytotoxicity Assay
To assess the cytotoxicity of 5-FU and As2O3, the MTT assay was performed to evaluate the viability of parent or 5-FU-resistant HCC cells. Briefly, the cells were cultured until they reached 80% confluence and then digested with trypsin, resulting in a single-cell suspension. The cells were inoculated into a 96-well plate (5 × 103 cells per well) and incubated overnight in DMEM containing 10% FBS. Following washing with PBS, the cells were treated with different concentrations of 5-FU (0, 0.5, 1, 5, 10, 50, 100, and 150 µM) and As2O3 (0, 0.5, 1, 2, 4, 8, 16, 32, 64, and 128 µM) for 72 h. After the treatment, the supernatant was discarded and the wells were washed with 1X PBS. Then, 10 µL of MTT reagent (5 mg/mL) was added to each well and the cells were incubated in a CO2 incubator for 4 h. After incubation, 100 µL of DMSO was added to dissolve the formazan crystals and the absorbance was determined at 560 nm using a microplate reader (Promega, Wisconsin, USA). The formula used to assess cell viability was:
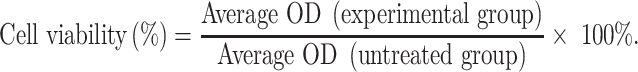 |
Cell viability was plotted against As2O3 concentration, and half-maximal inhibitory concentration (IC50) was calculated.
Cell Cycle Assay
To confirm whether As2O3 inhibits cell cycle progression, the infected 5-FU-HCC cells (2 × 105 cells/well) were inoculated in six-well plates and incubated overnight. Then, the cells were treated with or without 20 µM As2O3 for 24 h. Immediately following the incubation, the cells were trypsinized, centrifuged at 300 g for 5 min, washed with sterile PBS, and fixed for 3 h at −20 °C in 70% ethanol. The incubation was followed by centrifugation and washing with sterile PBS. About 200 µL of the cell cycle reagent was then added, followed by 30 min of incubation in the dark.
Protein Extraction and Western Blotting
Protein extraction was performed as previously described.22 Briefly, the culture medium was removed and the cells were lysed using the lysing solution. Protein concentration was determined using a commercial bicinchoninic acid assay kit (Thermo Fisher Scientific). Then, 10 g of sample was added to the gel well. Proteins separated using SDS-PAGE were transferred to the PVDF membrane, and the membrane was blocked with 5% milk powder mixed with the PBS/0.05% Tween-20 solution for 60 min. The samples were then incubated with a primary antibody overnight at 4 °C. The next day, the samples were incubated with a secondary antibody for an hour at room temperature. An enhanced chemiluminescence method was used to analyze the membrane. Data shown are averages of at least three independent experiments.
Cell Migration and Invasion Assays
As described previously,23 a cell migration assay was performed using 24-well plates, 6.5 mm chambers, and 8 μm wells (Corning Corporation, USA). Lower chambers were filled with DMEM containing 20% FBS (600 μL). The cells were digested and inoculated in DMEM containing 5% FBS at an adjusted concentration of 1×105 cells/mL. A total of 100 μL of this cell suspension was added to the upper chamber, and the membrane was carefully placed facing down on top of the cells. The cells were then fixed in 4% paraformaldehyde for 24 h at 37 °C. The cells were counted using a microscope by wiping the upper cell chamber with a cotton swab and staining the cells with 0.1% crystal violet solution.
Invasion chambers (BD Biosciences) were coated with Matrigel with a pore size of 8.0 μm in 24-well plates. Matrigel was situated facing the upper compartment and the sample was added to the lower compartment. Re-suspended tumor cells were added to the upper ventricle, where each group induced the invasive cells to penetrate the membrane. The results were evaluated after 24 h.
In vivo Tumor Xenograft Study
Four-week-old BALB/c-nu/nu nude mice (random number of males and females) were provided by the Shanghai Experimental Animal Center. All animal experiments were conducted in accordance with the ethical standards and national guidelines and approved by the Ethics Committee of the First Affiliated Hospital, Zhengzhou University.
In total, 40 mice weighing 20 to 25 g were maintained under standard conditions (12 h of light and darkness at 25 °C and 40% relative humidity). The mice received a subcutaneous injection of Hep3B or different infected Hep3B/5-FU cells (2 × 107 cells/mouse). As2O3 (0.5 mg/kg) or saline was administered intraperitoneally every other day after cells subcutaneous injection one week later. The tumor volumes were calculated (length × width2 /2) every three days, up to day 28. After euthanizing the mice, the tumors were removed for further analysis.
Immunohistochemistry
Paraffin-embedded 4 μm tissue sections from the tumor xenografts were analyzed using immunohistochemistry as previously described.24 Briefly, the antigens were unmasked by boiling the specimens in sodium citrate buffer (10 mM) after deparaffinization and dehydration. The specimens were then blocked and incubated overnight at 4 °C with the primary antibody against Ki67 (Solarbio, Beijing, China). Then, a second wash with PBS was performed. Antibody binding was detected using Envision reagents (Solarbio, Beijing, China) following the manufacturer’s instructions.
Statistical Analysis
The experimental data were analyzed using GraphPad Prism software (version 8.0) and expressed as mean ± standard errors of the mean (SEM). One-way ANOVA was used to compare variables across multiple groups. A p-value less than 0.05 was considered statistically significant.
Results
Response to 5-FU and As2O3 Treatment of HCC Cell Lines
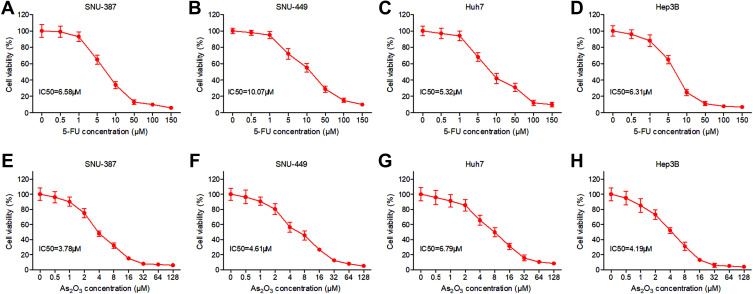
In order to investigate the effects of 5-FU on HCC cell growth, SNU-387, SNU-449, Huh7 and Hep3B cells were treated with 5-FU or As2O3 at increasing concentrations for 72 hours and the viability was analyzed with MTT. As shown in Figure 1A–D, 5-FU inhibited HCC cells in a dose-dependent manner. Its IC50 values were 6.58μM, 10.07μM, 5.32μM and 6.31μM for SNU-387, SNU-449, Huh7, and Hep3B cells, respectively. According to Figure 1E–G, As2O3 significantly inhibits the growth of HCC cells with increasing doses, with an IC50 value of 3.78μM for SNU-387, 4.61μM for SNU-449, 6.79μM for Huh7 and 4.19μM for Hep3B.
Figure 1.
Response to 5-FU and As2O3 treatment of HCC cell lines. SNU-387, SNU-449, Huh7 and Hep3B cells were treated with different concentrations of 5-FU (A–D) or As2O3 (E–H) for 72 hours and the viability was analyzed with MTT assay.
As2O3 in Combination with siNrf2 Decreased 5-FU-Resistant HCC Cell Viability
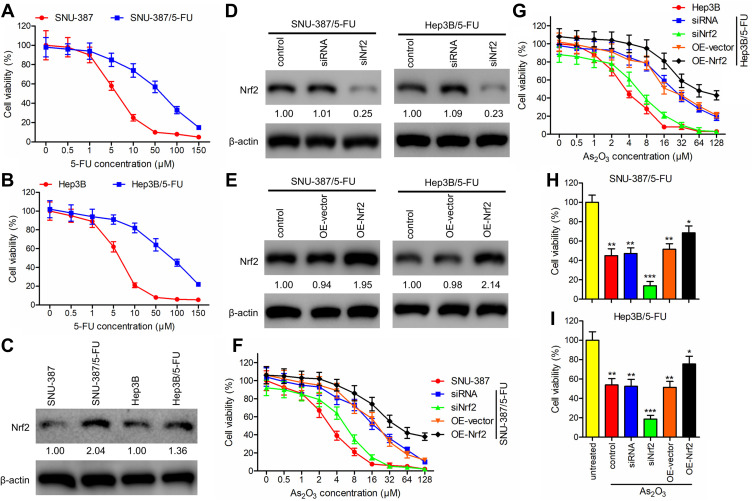
To investigate the influence of As2O3 on 5-FU resistance in HCC, the HCC cell lines SNU-387 and Hep3B were incubated with increasing concentrations of 5-FU to generate the 5-FU-resistant cell lines SNU-387/5-FU and Hep3B/5-FU. Cell viability of SNU-387/5-FU and Hep3B/5-FU lines exposed to increasing concentrations of 5-FU for 72 h was measured using the MTT assay. Compared to the parental cell lines, SNU-387/5-FU and Hep3B/5-FU were more resistant to 5-FU, with IC50 values of 61.12 μM and 85.50 μM, respectively, which were 9.8- and 13.2-fold higher than the IC50 values of the parental lines SNU-387 and Hep3B, respectively (6.25 μM and 6.48 μM, respectively) (Figure 2A and B).
Figure 2.
As2O3 in combination with siNrf2 decreased cell viability of 5-FU-resistant HCC cells. (A and B) Cell viability was assessed using the MTT assay in (A) SNU-387 and SNU-387/5-FU and (B) Hep3B and Hep3B/5-FU cells after incubation with increasing concentrations of 5-FU for 72 h. (C) Nrf2 expression was detected using Western blotting and expressed as a fold-change relative to the parental cell lines. β-actin was used as the internal reference. (D and E) Using lentiviral vectors carrying siRNA targeting Nrf2 (siNrf2) or the Nrf2 gene (OE-Nrf2), Nrf2 was knocked down or overexpressed, respectively, in SNU-387/5-FU and Hep3B/5-FU cells. Western blotting was performed to detect Nrf2 expression. (F and G) The impact of Nrf2 knockdown or overexpression combined with different concentrations of As2O3 on the growth of (F) SNU-387 and SNU-387/5-FU and (G) Hep3B and Hep3B/5-FU cells was studied using the MTT assay. (H and I) The viability of (H) SNU-387/5-FU and (I) Hep3B/5-FU cells treated with or without 20 μM As2O3 for 72 h was measured using the MTT assay. All data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; versus the untreated group.
Additionally, we evaluated the role of Nrf2 in 5-FU-resistant HCC cells. Western blotting analysis revealed that 5-FU-resistant cells expressed elevated levels of Nrf2 (Figure 2C). Additionally, we used siNrf2 and OE-Nrf2 to knock down and overexpress endogenous Nrf2, respectively, in 5-FU-resistant HCC cells (Figure 2D and E). The MTT assay showed an As2O3-concentration-dependent reduction in the viability of HCC cells. Moreover, 5-FU-resistant HCC cells showed lower As2O3 sensitivity than their parental cells. In 5-FU-resistant cell lines, Nrf2 knockdown partially reversed As2O3 resistance, and Nrf2 overexpression increased it (Figure 2F and G). When As2O3 treatment was combined with Nrf2 knockdown, SNU-387/5-FU and Hep3B/5-FU cells were significantly less viable (Figure 2H and I). The results suggested that As2O3 combined with siNrf2 may be more effective in treating cancer than As2O3 alone.
As2O3 in Combination with siNrf2 Inhibited the Migration and Invasion of 5-FU-Resistant HCC Cells by Inactivating HIF-1α Signaling
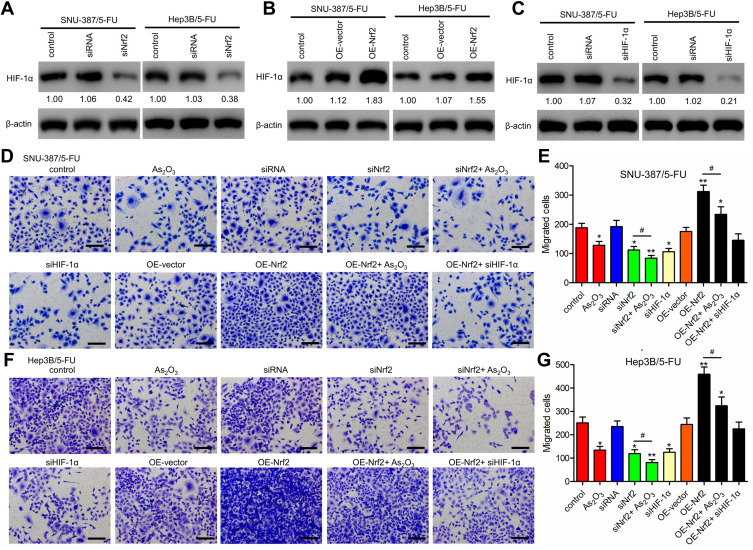
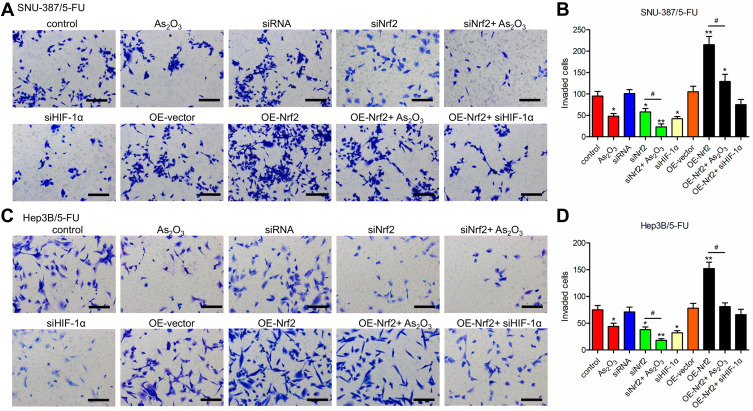
Western blotting analysis showed that HIF-1α levels were downregulated in siNrf2 5-FU-resistant cells and upregulated in OE-Nrf2 5-FU-resistant cells (Figure 3A and B). To further examine the effects of HIF-1α on 5-FU-resistant cells, we stably knocked down endogenous HIF-1α using siHIF-1α (Figure 3C). The transwell assay showed that As2O3, siNRF2, and siHIF-1α inhibited the migration (Figure 3D–G) and invasion (Figure 4) of SNU-387/5-FU and Hep3B/5-FU cells compared to the control. Also, siNrf2 treatment combined with As2O3 inhibited cell migration and invasion more than siNrf2 or As2O3 alone. On the other hand, OE-Nrf2 increased the migration and invasion on 5-FU-resistant cells, and this effect was mitigated by As2O3 and siHIF-1α. The results indicated that As2O3 combined with siNrf2 effectively inhibited cell migration and invasion, which was dependent on inactivating HIF-1α.
Figure 3.
Nrf2 knockdown inhibited the migration of 5-FU-resistant HCC cells by inactivating HIF-1α signaling. (A and B) Western blotting analysis of HIF-1α levels in Nrf2-knockdown cells (A), Nrf2-overexpressed cells (B), and control cells. (C) HIF-1α was knocked down in SNU-387/5-FU and Hep3B/5-FU cells. (D–G) Migration was determined using the Transwell assay in (D and E) SNU-387/5-FU and (F and G) Hep3B/5-FU cells. Scale bar = 100 μm. All data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; versus the control group. #P < 0.05 versus the indicated groups.
Figure 4.
As2O3 in combination with siNrf2 inhibited the invasion of 5-FU-resistant HCC cells. The invasion was determined using the Transwell assay in (A and B) SNU-387/5-FU and (C and D) Hep3B/5-FU cells. Scale bar = 100 μm. All data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; versus the control group. #P < 0.05; versus the indicated groups.
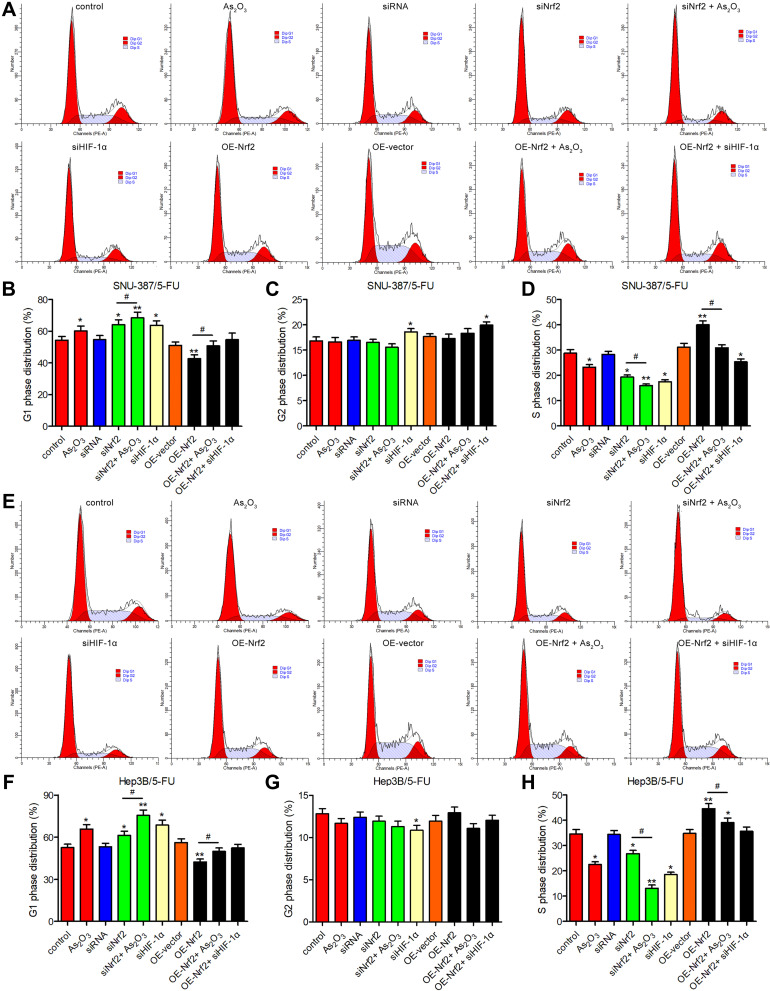
The Combination of As2O3 and siNrf2 Induced G1 Cell Cycle Arrest and Enhanced Apoptosis in 5-FU-Resistant HCC Cells
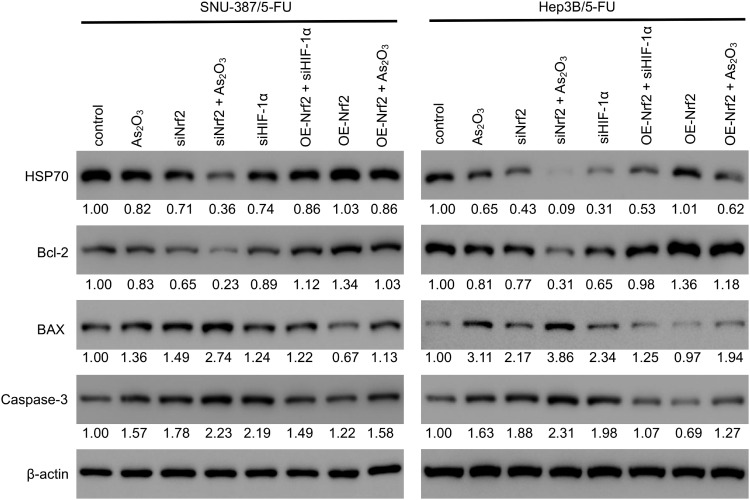
The evaluation of the cell cycle using flow cytometry revealed that a higher number of cells in the G1 phase and a lower number of cells in the S phase were observed in the siNrf2-treated cells with or without As2O3 treatment than in the control cells, while the opposite results were observed in the OE-Nrf2-treated cells, and As2O3 treatment and siHIF-1α reversed the effect of OE-Nrf2 (Figure 5). However, neither siNrf2 nor OE-Nrf2 influenced the number of cells in the G2 phase. Consistently, Nrf2 knockdown combined with As2O3 significantly reduced the levels of 70 kDa heat-shock protein (HSP70). The anti-apoptotic protein Bcl-2, the pro-apoptotic protein BAX, and the apoptotic factor caspase-3 were also analyzed (Figure 6). Compared to As2O3 or siNrf2 alone, combined As2O3 decreased Bcl-2 abundance, enhanced BAX expression, and increased caspase-3 abundance, while OE-Nrf2-treated cells showed the opposite results, and treatment with As2O3 and siHIF-1α reversed the effect on OE-Nrf2-treated cells. The results indicated that silencing Nrf2 in combination with As2O3 treatment inhibited the proliferation and promoted apoptosis of 5-FU-resistant HCC cells.
Figure 5.
The combination of As2O3 and siNrf2 induced G1 cell cycle arrest of 5-FU-resistant HCC cells. The cell cycle was analyzed using flow cytometry after treatment with or without 20 μM As2O3 for 24 h in (A–D) SNU-387/5-FU and (E–H) Hep3B/5-FU cells. All data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; versus the control group. #P < 0.05; versus the indicated groups.
Figure 6.
Nrf2 expression was associated with apoptosis of 5-FU-resistant HCC cells targeting the HIF-1α/HSP70 pathway. The levels of HSP70 and apoptosis markers (Bcl-2, BAX, and cleaved caspase-3) were measured using Western blotting in SNU-387/5-FU and Hep3B/5-FU cells. The protein levels of the genes of interest were normalized to that of β-actin and expressed as a fold-change relative to the control group.
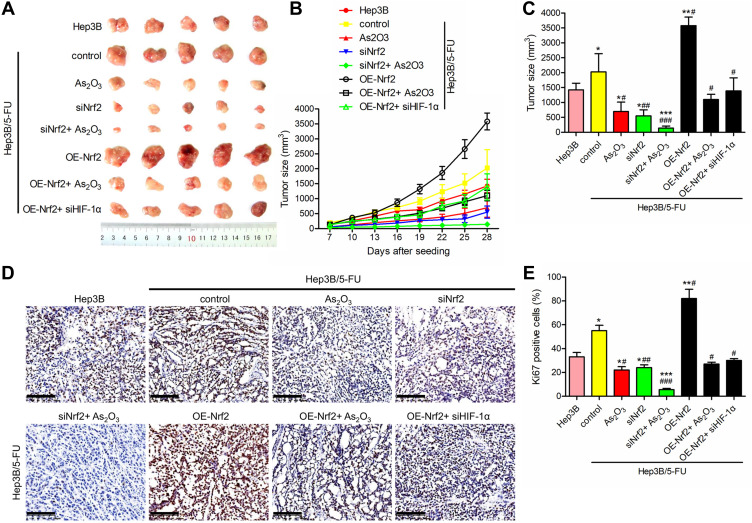
As2O3 in Combination with siNrf2 Suppressed 5-FU Resistance in vivo
To verify the therapeutic effect of As2O3 in combination with siNrf2 on Hep3B/5-FU growth in vivo, we established subcutaneous transplantation tumor models using Hep3B and Hep3B/5-FU cells with or without stable Nrf2 or HIF-1α knockdown or Nrf2 overexpression in nude mice. As2O3 or saline was administered to the tumor-bearing mice one week after tumor implantation. Figure 7A shows the tumor images. siNrf2 Hep3B/5-FU cell transplantation with As2O3 treatment reduced tumor proliferation and volume significantly (Figure 7B and C). When Nrf2 was overexpressed, tumor growth was accelerated, while As2O3 treatment diminished this effect. A significant decrease in Ki-67-positive cells was observed in tumors containing siNrf2-treated cells after As2O3 was administered (Figure 7D and E). These findings suggested that Nrf2 knockdown combined with As2O3 is an effective treatment for 5-FU-resistant HCC in vivo.
Figure 7.
As2O3 in combination with siNrf2 enhanced the suppression of 5-FU resistance in vivo. Nude mice were injected subcutaneously with Hep3B cells or transfected Hep3B/5-FU cells (2 × 107 cells/mouse) and administered 0.5 mg/kg As2O3 or saline once every other day intraperitoneally. (A) Images of the representative tumor xenografts were obtained 28 days after tumor implantation. (B) The tumor volume was measured every three days after day 7. (C) The tumor volume was measured 28 days after implantation. (D) Representative images of Ki-67 IHC staining, scale bar = 100 μm. (E) The percentage of Ki-67-positive cells. n = 5 for each group. All data are expressed as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; versus the Hep3B group. #P < 0.05; ##P < 0.01; ###P < 0.001; versus the Hep3B/5-FU control group.
Discussion
Currently, the anti-cancer treatment methods include surgical treatment, chemotherapy, and radiotherapy.25 Advanced cancer metastasis and recurrence are often caused by cancer cells that have developed resistance to the chemotherapy drugs.26 Drug resistance in cancer is one of the major obstacles to successful cancer treatment.27 So, novel chemicals inhibiting drug-resistant cancers are needed. It has been shown that As2O3 is effective not only in treating chronic lymphocytic leukemia and acute myeloid leukemia, but also in treating some solid tumors, such as lung, ovarian, breast, and cervical cancer.28–30 As2O3 is a potent angiogenesis inhibitor that can damage mitochondria, leading to oxidative stress, reversing drug resistance in cancers. In agreement with the earlier study,31 we found that As2O3 shows dose-dependent effects. As2O3 inhibits cell growth in human drug-resistant tumor cell lines and large doses of As2O3 were required to treat drug-resistant cancers. Therefore, the development of methods that may improve the therapeutic efficacy while minimizing toxicity is a key topic in chemotherapy research.
According to Wang et al, locoregional therapy (LRT) with As2O3 treatment prolonged survival times for patients with primary HCC.32 Furthermore, although As2O3 as a single agent showed low efficacy in Phase II trials,33 its combination with other drugs may be more effective in treating HCC.34 A significant correlation was demonstrated by Zhang et al between the expression level of Nrf2 and tumor differentiation, metastasis, and size of the tumor.35 And high Nrf2 expression correlates with tumor chemotherapy resistance.36 In this study, it was found that the Nrf2 expression level of drug-resistant cells was higher than that of parent cell lines. Moreover, in drug-resistant cell lines, As2O3 combined with siNrf2 at a relatively low concentration can play a role in tumor inhibition. The present study demonstrated that As2O3 in combination with siNrf2 significantly inhibited the proliferation of SNU-387/5-FU and Hep3B/5-FU cells via G1-phase arrest as well as induction of apoptosis, as evidenced using flow cytometry analysis. Also, As2O3 combined with siNrf2 significantly inhibited the migration and invasion of SNU-387/5-FU and Hep3B/5-FU cells more than As2O3 or siNrf2 alone.
Studies have also shown that solid tumor cells are insensitive to chemotherapy drugs under hypoxic conditions, which may be an important cause of drug resistance in tumor cells. HIF-1α is a nuclear transcription factor that plays an important role in hypoxia-mediated multidrug resistance. The mechanism underlying the involvement of HIF-1α in hypoxia-mediated multidrug resistance of tumor cells involves a reduction in chemotherapy drug accumulation in the tumor cells and inhibition of apoptosis induced by chemotherapy drugs. However, several HIF-1α inhibitors have off-target side effects or adverse pharmacological properties, prompting researchers to search for more effective HIF-1α inhibitors.37–39 Nrf2 is a critical regulator of the antioxidant response. Previous studies elucidating the role of Nrf2 in hypoxia-induced drug resistance have shown that a direct interaction between Nrf2 and HIF-1α enhancer plays an important role in chemotherapy resistance in the hypoxic tumor microenvironment.40,41 Specifically, this study inhibited HIF-1α level by down-regulating Nrf2 expression. A recent study in cultured HCC cells found that Nrf2 directly binds to the oxygen dependent degradation (ODD) domain of HIF-1α, which limits prolyl hydroxylase domain proteins (PHDs) mediated hydroxylation of HIF-1α, thereby preventing binding of hydroxylated HIF-1α to von-Hippel-Lindau (VHL) for proteasomal degradation, which represents breaking NRF2-HIF-1α alliance may provide more efficient therapeutic strategies than targeting each transcription factor alone.42
After the administration of chemotherapy, the Bcl-2/BAX ratio determines whether tumor cells are sensitive to apoptosis.43,44 In the study, treatment with As2O3 in combination with siNrf2 resulted in significant Bcl-2 downregulation and simultaneous BAX upregulation in HCC cells. An increased Bax/Bcl-2 ratio leads to enhanced apoptosis induced by cytokines and chemotherapeutic agents. In addition, caspase-3 expression in the present study increased in SNU-387/5-FU and Hep3B/5-FU cells treated with As2O3 in combination with siNrf2, suggesting that As2O3 in combination with siNrf2 led to caspase-mediated apoptosis. Nevertheless, clinical studies combining As2O3 with 5-FU and targeted Nrf2 are necessary to clarify the clinical efficacy of As2O3 in future studies.
Conclusion
We demonstrated for the first time that siNrf2 greatly improved the inhibitory effect of As2O3 on the growth, invasion, and migration of drug-resistant liver cancer cells, which was also verified in vivo. Further research indicated that this combined action inhibited HIF-1α and reduced the effect of hypoxia-mediated cellular drug resistance.
Funding Statement
This study was supported by National Natural Science Foundation of Henan Province (No. 202300410361).
Data Sharing Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics Approval
All animal experiments were conducted in accordance with the ethical standards and national guidelines and approved by the Ethics Committee of the First Affiliated Hospital, Zhengzhou University.
Author Contributions
All authors made a significant contribution to the work whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed to submit to the current journal; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Zhou HM, Zhang JG, Zhang X, Li Q. Targeting cancer stem cells for reversing therapy resistance: mechanism, signaling, and prospective agents. Signal Transduct Target Ther. 2021;6(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assaraf YG, Brozovic A, Goncalves AC, et al. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist Updat. 2019;46:100645. doi: 10.1016/j.drup.2019.100645 [DOI] [PubMed] [Google Scholar]
- 3.Kartal-Yandim M, Adan-Gokbulut A, Baran Y. Molecular mechanisms of drug resistance and its reversal in cancer. Crit Rev Biotechnol. 2016;36(4):716–726. doi: 10.3109/07388551.2015.1015957 [DOI] [PubMed] [Google Scholar]
- 4.Baguley BC. Multidrug resistance in cancer. Methods Mol Biol. 2010;596:1–14. [DOI] [PubMed] [Google Scholar]
- 5.Lee HA, Chu KB, Moon EK, Kim SS, Quan FS. Sensitization to oxidative stress and G2/M cell cycle arrest by histone deacetylase inhibition in hepatocellular carcinoma cells. Free Radic Biol Med. 2020;147:129–138. doi: 10.1016/j.freeradbiomed.2019.12.021 [DOI] [PubMed] [Google Scholar]
- 6.Jin M, Yang Y, Dai Y, et al. 27-hydroxycholesterol is a specific factor in the neoplastic microenvironment of HCC that causes MDR via GRP75 regulation of the redox balance and metabolic reprogramming. Cell Biol Toxicol. 2021. doi: 10.1007/s10565-021-09607-y [DOI] [PubMed] [Google Scholar]
- 7.Thomas M. Molecular targeted therapy for hepatocellular carcinoma. J Gastroenterol. 2009;44(Suppl 19):136–141. doi: 10.1007/s00535-008-2252-z [DOI] [PubMed] [Google Scholar]
- 8.Lee YG, Jeon TI. Modulation of the autophagy-lysosomal pathway in hepatocellular carcinoma using small molecules. Molecules. 2020;25(7):1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui F, Xu C, Xu X, et al. What is the most suitable agent combined with apatinib for transarterial chemoembolization treatment in advanced hepatocellular carcinoma patients? A systematic review and network meta-analysis. Front Oncol. 2022;12:887332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo J, Huang L. Formulation of two lipid-based membrane-core nanoparticles for FOLFOX combination therapy. Nat Protoc. 2022;17(8):1818–1831. doi: 10.1038/s41596-022-00698-3 [DOI] [PubMed] [Google Scholar]
- 11.Lai Z, He M, Bu X, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: a biomolecular exploratory, phase II trial. Eur J Cancer. 2022;174:68–77. doi: 10.1016/j.ejca.2022.07.005 [DOI] [PubMed] [Google Scholar]
- 12.Ganan-Gomez I, Wei Y, Yang H, Boyano-Adanez MC, Garcia-Manero G. Oncogenic functions of the transcription factor Nrf2. Free Radic Biol Med. 2013;65:750–764. doi: 10.1016/j.freeradbiomed.2013.06.041 [DOI] [PubMed] [Google Scholar]
- 13.Sajadimajd S, Khazaei M. Oxidative stress and cancer: the role of Nrf2. Curr Cancer Drug Targets. 2018;18(6):538–557. doi: 10.2174/1568009617666171002144228 [DOI] [PubMed] [Google Scholar]
- 14.Buendia I, Michalska P, Navarro E, Gameiro I, Egea J, Leon R. Nrf2-ARE pathway: an emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther. 2016;157:84–104. doi: 10.1016/j.pharmthera.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 15.Sebestyén A, Kopper L, Dankó T, Tímár J. Hypoxia signaling in cancer: from basics to clinical practice. Pathol Oncol Res. 2021;27:1609802. doi: 10.3389/pore.2021.1609802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L, Zhao Z, Yin Q, Zhang X. TTB protects astrocytes against oxygen-glucose deprivation/reoxygenation-induced injury via activation of Nrf2/HO-1 signaling pathway. Front Pharmacol. 2019;10:792. doi: 10.3389/fphar.2019.00792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duan X-H, Li H, Ren J-Z, et al. Hepatic arterial chemoembolization with arsenic trioxide eluting CalliSpheres microspheres versus lipiodol emulsion: pharmacokinetics and intratumoral concentration in a rabbit liver tumor model. Cancer Manag Res. 2019;11:9979–9988. doi: 10.2147/CMAR.S199188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan X, Li H, Han X, et al. Antitumor properties of arsenic trioxide-loaded CalliSpheres® microspheres by transarterial chemoembolization in VX2 liver tumor rabbits: suppression of tumor growth, angiogenesis, and metastasis and elongation of survival. Am J Transl Res. 2020;12(9):5511–5524. [PMC free article] [PubMed] [Google Scholar]
- 19.Duan X, Zhao G, Han X, et al. Arsenic trioxide-loaded CalliSpheres: in vitro study of drug release and antitumor activity, and in vivo study of pharmacokinetics, treatment efficacy and safety in liver cancer. Oncol Rep. 2021;46(1). doi: 10.3892/or.2021.8075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Zhao X, Zhu D, et al. HIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment. J Exp Clin Cancer Res. 2017;36(1):60. doi: 10.1186/s13046-017-0533-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pu Y, Yan D, Tu L, et al. CDK inhibition reverses acquired 5-fluorouracil resistance in hepatocellular carcinoma cells. Dis Markers. 2022;2022:6907057. doi: 10.1155/2022/6907057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Li Q, Chen XZ. Detecting protein-protein interactions by far Western blotting. Nat Protoc. 2007;2(12):3278–3284. doi: 10.1038/nprot.2007.459 [DOI] [PubMed] [Google Scholar]
- 23.Wan G, Liu Y, Zhu J, et al. SLFN5 suppresses cancer cell migration and invasion by inhibiting MT1-MMP expression via AKT/GSK-3beta/beta-catenin pathway. Cell Signal. 2019;59:1–12. doi: 10.1016/j.cellsig.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 24.Serna G, Simonetti S, Fasani R, et al. Sequential immunohistochemistry and virtual image reconstruction using a single slide for quantitative KI67 measurement in breast cancer. Breast. 2020;53:102–110. doi: 10.1016/j.breast.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saraswathy M, Gong S. Different strategies to overcome multidrug resistance in cancer. Biotechnol Adv. 2013;31(8):1397–1407. doi: 10.1016/j.biotechadv.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 26.Bijnsdorp IV, Giovannetti E, Peters GJ. Analysis of drug interactions. Methods Mol Biol. 2011;731:421–434. [DOI] [PubMed] [Google Scholar]
- 27.Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575(7782):299–309. doi: 10.1038/s41586-019-1730-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wahiduzzaman M, Ota A, Hosokawa Y. Novel mechanistic insights into the anti-cancer mode of arsenic trioxide. Curr Cancer Drug Targets. 2020;20(2):115–129. doi: 10.2174/1568009619666191021122006 [DOI] [PubMed] [Google Scholar]
- 29.Kozono S, Lin YM, Seo HS, et al. Arsenic targets Pin1 and cooperates with retinoic acid to inhibit cancer-driving pathways and tumor-initiating cells. Nat Commun. 2018;9(1):3069. doi: 10.1038/s41467-018-05402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong Y, Li X. Prospect of research and clinical application of arsenic compounds in chemotherapy for gynecological malignant tumors. Gynecol Obstet Clin Med. 2022;2(1):23–28. doi: 10.1016/j.gocm.2022.01.001 [DOI] [Google Scholar]
- 31.Zhang X, Hu B, Sun YF, et al. Arsenic trioxide induces differentiation of cancer stem cells in hepatocellular carcinoma through inhibition of LIF/JAK1/STAT3 and NF-kB signaling pathways synergistically. Clin Transl Med. 2021;11(2):e335. doi: 10.1002/ctm2.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Liu Y, Wang X, et al. Randomized clinical control study of locoregional therapy combined with arsenic trioxide for the treatment of hepatocellular carcinoma. Cancer. 2015;121(17):2917–2925. doi: 10.1002/cncr.29456 [DOI] [PubMed] [Google Scholar]
- 33.Lin CC, Hsu C, Hsu CH, Hsu WL, Cheng AL, Yang CH. Arsenic trioxide in patients with hepatocellular carcinoma: a phase II trial. Invest New Drugs. 2007;25(1):77–84. doi: 10.1007/s10637-006-9004-9 [DOI] [PubMed] [Google Scholar]
- 34.Sönksen M, Kerl K, Bunzen H. Current status and future prospects of nanomedicine for arsenic trioxide delivery to solid tumors. Med Res Rev. 2022;42(1):374–398. doi: 10.1002/med.21844 [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Zhang C, Zhang L, et al. Nrf2 is a potential prognostic marker and promotes proliferation and invasion in human hepatocellular carcinoma. BMC Cancer. 2015;15:531. doi: 10.1186/s12885-015-1541-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong YB, Kang HJ, Kwon SY, et al. Nuclear factor (erythroid-derived 2)-like 2 regulates drug resistance in pancreatic cancer cells. Pancreas. 2010;39(4):463–472. doi: 10.1097/MPA.0b013e3181c31314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue X, Zhao NY, Yu HT, et al. Discovery of novel inhibitors disrupting HIF-1alpha/von Hippel-Lindau interaction through shape-based screening and cascade docking. PeerJ. 2016;4:e2757. doi: 10.7717/peerj.2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin D, Wu J. Hypoxia inducible factor in hepatocellular carcinoma: a therapeutic target. World J Gastroenterol. 2015;21(42):12171–12178. doi: 10.3748/wjg.v21.i42.12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takaki H, Hirata Y, Ueshima E, et al. Hepatic artery embolization enhances expression of programmed cell death 1 ligand 1 in an orthotopic rat hepatocellular carcinoma model: in vivo and in vitro experimentation. J Vasc Interv Radiol. 2020;31(9):1475–1482 e1472. doi: 10.1016/j.jvir.2020.03.023 [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Zhang J, Lu Y, Luo Q, Zhu L. Nuclear respiratory factor-1 (NRF-1) regulated hypoxia-inducible factor-1alpha (HIF-1alpha) under hypoxia in HEK293T. IUBMB Life. 2016;68(9):748–755. doi: 10.1002/iub.1537 [DOI] [PubMed] [Google Scholar]
- 41.Waghela BN, Vaidya FU, Pathak C. Upregulation of NOX-2 and Nrf-2 promotes 5-fluorouracil resistance of human colon carcinoma (HCT-116) cells. Biochemistry. 2021;86(3):262–274. doi: 10.1134/S0006297921030044 [DOI] [PubMed] [Google Scholar]
- 42.Zheng J, Kim S-J, Saeidi S, et al. Overactivated NRF2 induces pseudohypoxia in hepatocellular carcinoma by stabilizing HIF-1α. Free Radic Biol Med. 2022. doi: 10.1016/j.freeradbiomed.2022.11.039 [DOI] [PubMed] [Google Scholar]
- 43.Mackey TJ, Borkowski A, Amin P, Jacobs SC, Kyprianou N. bcl-2/bax ratio as a predictive marker for therapeutic response to radiotherapy in patients with prostate cancer. Urology. 1998;52(6):1085–1090. doi: 10.1016/S0090-4295(98)00360-4 [DOI] [PubMed] [Google Scholar]
- 44.Mirjolet JF, Barberi-Heyob M, Didelot C, et al. Bcl-2/Bax protein ratio predicts 5-fluorouracil sensitivity independently of p53 status. Br J Cancer. 2000;83(10):1380–1386. doi: 10.1054/bjoc.2000.1455 [DOI] [PMC free article] [PubMed] [Google Scholar]