FIGURE 1.
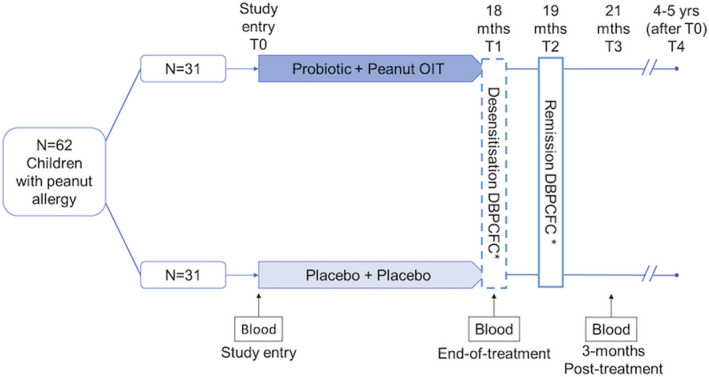
PPOIT‐001 randomized trial design. *DBPCFC, double‐blind placebo‐controlled food challenge. n = 62 children aged 1–10 years with peanut allergy were randomized 1:1 to receive probiotic and peanut oral immunotherapy (PPOIT) or placebo for 18 months. A double‐blind placebo‐controlled food challenge (DBPCFC; cumulative 4 g peanut protein) was performed at end‐of‐treatment (T1) and at 2–6‐week post‐treatment (T2). Subjects who failed the T1 DBPCFC were classified as allergic. Subjects who passed both the T1 and T2 DBPCFC were classified as having attained remission/SU. Blood samples were collected at various times. This study included n = 16 PPOIT‐treated children who attained remission/SU and n = 16 placebo‐treated children who remained allergic. PBMC collected from these subjects at baseline (T0), end‐of‐treatment (T1), and 3‐month post‐treatment (T3) underwent transcriptomic profiling
