Abstract
Background
Delaying cord clamping (CC) for 3‐5 minutes reduces iron deficiency and improves neurodevelopment. Data on the effects of CC beyond 3 minutes in relation to short‐term neonatal outcomes and maternal risk of postpartum hemorrhage are scarce.
Methods
This was a prospective observational study performed in two delivery departments. Pregnant women with vaginal deliveries were included. Time to CC, estimated postpartum blood loss, and perinatal data were recorded. Spearman's correlation analysis and comparisons between newborns clamped before and after 3 minutes were performed.
Results
In total, 904 dyads were included. The mean gestational age ± standard deviation was 40.1 ± 1.2 weeks. CC was performed at a median time of 6 minutes (range 0‐23.5). Apgar scores at 5 and 10 minutes were positively correlated with time to CC (correlation coefficient .140, P < .001 and .161, < .001). There was no correlation between CC time and bilirubin level (correlation coefficient .021, P = .54). The median postpartum blood loss was 300 mL (70‐2550 mL), with a negative correlation between CC time and postpartum blood loss (−0.115, P = .001). The postpartum blood loss was larger in the group clamped at ≤3 minutes (median [interquartile range] 400 mL [300‐600] vs 300 mL [250‐450], [P = .003]].
Conclusions
Umbilical CC times beyond 3 minutes in vaginal deliveries were not associated with negative short‐term outcomes in newborns and were associated with a smaller maternal postpartum blood loss. Although CC time as long as 6 minutes could be considered as safe, further research is needed to decide the optimal timing.
Keywords: cord clamping, hyperbilirubinemia, postpartum hemorrhage, umbilical cord
1. BACKGROUND
By delaying cord clamping (CC) for 3‐5 minutes, term infants receive a placental transfusion of 25%‐35% of their total blood volume. 1 , 2 Beneficial effects after delayed CC include reduced iron deficiency at 3‐8 months, improved neurodevelopment at 12 and 48 months, 3 , 4 , 5 , 6 , 7 and improved brain myelin content during infancy. 8 , 9 In preterm infants, delayed CC is associated with reduced mortality by approximately 30%. 10 , 11
However, delayed CC has been proposed to be associated with an increased risk of hyperbilirubinemia requiring phototherapy. 12 , 13 In the 2020 revised committee opinion with respect to CC, the American College of Obstetricians and Gynecologists reported a small increase in the incidence of jaundice that required phototherapy in term infants after delayed CC. 13 It is not known whether CC delayed beyond 3 minutes is associated with other neonatal complications. Early CC (<60 seconds) has been part of active management and is believed to reduce the risk of postpartum hemorrhage (PPH). Consequently, there have been concerns that delayed CC could increase the risk of PPH 14 and affect the timing of oxytocin administration. 15
Despite these proposed risks, we found in a nationwide telephone survey that most midwives did not clamp the umbilical cord at the recommended time of 2‐3 minutes but waited for cessation of pulsations or delivery of the placenta. There was also variation in the timing of oxytocin administration. 16
The objective of our study was to explore whether CC delayed beyond 3 minutes was associated with increased risks for newborns or mothers.
2. METHODS
This is an observational cohort study conducted in the two delivery departments at the Hospital of Halland in the cities of Halmstad and Varberg, Sweden, from April 28, 2017 to March 18, 2018. Pregnant women were considered eligible if they were expecting a vaginal delivery at ≥35 + 0 weeks of gestation and were able to understand Swedish well enough to comprehend the information given about the study. Reasons for exclusion were incomplete or neglected data sheets, time to CC not measured, and unplanned cesarean birth. Information brochures and inquiries for consent were handed out by registered midwives at the antenatal care units or when the woman arrived at the delivery department. Oral and written consent was obtained from the mother. The study was approved by the Regional Ethical Review Board at Lund University (2016/1052).
The purpose of the study was to investigate the association between the timing of umbilical CC and neonatal and maternal short‐term outcomes. Specifically, we aimed to investigate whether CC beyond 3 minutes was associated with any increased risk for newborns or mothers by analyzing the primary outcomes of the need for phototherapy, admission to the neonatal ward, and maternal postpartum blood loss in relation to umbilical CC time. Furthermore, we analyzed additional neonatal short‐term outcomes, including Apgar score, neonatal adverse events, and transcutaneous bilirubin at metabolic screen in relation to CC time. The association between oxytocin administration and postpartum blood loss was also analyzed.
2.1. Study protocol and data collection
At the time of delivery, a midwife or assistant nurse registered the time of umbilical CC with a stopwatch. Oxytocin (5 IU, 8.3 μg/mL) administration was noted as amount (mL), “before CC,” “after CC,” or “not administered.” If performed, fundal massage was documented. The midwife or assistant nurse recorded the estimated maternal postpartum blood loss within 2 hours after childbirth by weighing pads, sheets, and other textiles and by measuring the volume of blood collected in a pan. Apgar scores were assessed by the midwife. Signs of respiratory distress, infection, hypoglycemia, and/or admission to the neonatal ward were recorded on a data sheet by staff during the infant's admission to the nursery. At the time of metabolic screening (≥48 hours after delivery), midwives measured transcutaneous bilirubin using the Dräger Jaundice Meter JM‐105 (Dräger Medical) and registered if the newborn had been admitted to the neonatal ward, if blood samples other than metabolic screen had been taken, or if phototherapy had to be performed. Decisions on initiating phototherapy were made by the attending pediatrician after an adapted version of the Bhutani nomogram. 17
At data analysis, PPH was defined as >500 mL maternal postpartum blood loss. The risk of developing significant neonatal hyperbilirubinemia was assessed by using a predictive nomogram for transcutaneous bilirubin up to 72 hours after birth, as proposed by Varavarigou et al. 18 All newborns were categorized into low, intermediate, or high risk of hyperbilirubinemia, according to the Varavarigou nomogram.
Baseline data about the mother and her pregnancy were acquired from the antenatal records, and together with newborn data, these were entered into a database.
Study size was decided in the protocol as inclusion of 500 pregnancies from each of the two delivery departments. As we had preliminary data on a large variety of CC practices among midwives, we wanted to ensure a sample large enough to capture this variation.
2.2. Statistical analysis
Data are presented as numbers (percentage), medians (minimum–maximum), or means ± standard deviation as appropriate by distribution. Comparisons between the two groups were analyzed by Student t test or Mann‐Whitney U test with means or medians and confidence intervals or minimum‐maximum range, as appropriate according to the distribution of the variables. Group comparisons of categorical variables were compared by Fisher chi‐squared2 exact test. ANOVA was performed when comparing more than two groups, and to adjust for multiple comparisons, Bonferroni adjustment was chosen. Correlations were analyzed by Pearson's r or Spearman's rho as appropriate by the distribution of the variable.
Variables identified as correlated with the primary outcomes were entered into logistic regression models. Variables with P < .10 in the correlation analysis were included in the models.
The logistic regression models were validated according to common practices: no or few outliers were observed and no multicollinearity was found. Models from the regression analyses were described by the following parameters; chi‐square (degrees of freedom), P value, and Model R 2 (Cox and Snell and Nagelkerke) B, the direction of the specified independent variable in the model; odds ratio (95% Confidence interval [CI]) for B; P value, the significance of the specified independent variable in the model.
P values below or equal to .05 were considered significant.
3. RESULTS
3.1. Study cohort
Participants were recruited from April 28, 2017 to February 15, 2018 at Halmstad and from June 2, 2017 to March 2, 2018 at Varberg. During this time, 3698 newborns were delivered in the two hospitals, and 3147 met the defined inclusion criteria (gestational age ≥ 35 weeks, planned vaginal delivery). A total of 2147 women were not approached, did not want to join the study, or did not understand Swedish sufficiently. Reasons were not documented according to ethical regulations. Finally, out of 1000 women recruited from the maternity wards, recordings of 96 infants had to be excluded from analysis, 49 from Halmstad and 47 from Varberg (Figure 1). Reasons for exclusion were incomplete or neglected data sheets (n = 85), time to CC not measured (n = 8), and unplanned cesarean sections (n = 3). Thus, a total of 904/1000 (90.4%) newborns were included in the analysis, among whom 463 (51.2%) were boys. It was the first pregnancy in 453 (50.1%) cases, the second in 309 (34.2%), and the third in 106 (11.7%). Maternal and neonatal characteristics are shown in Table 1. Complete baseline data about the mother and the pregnancy were available from the antenatal records in 658 (72.8%) cases. When the mother resided outside the county of Halland (246 cases, 27.2%), maternal weight and length at first antenatal visit were not available. Data on prespecified outcomes are presented relating to the timing of umbilical CC in Table 2. As prespecified in the protocol, we divided newborns into groups with umbilical CC time ≤3 minutes and >3 minutes.
FIGURE 1.
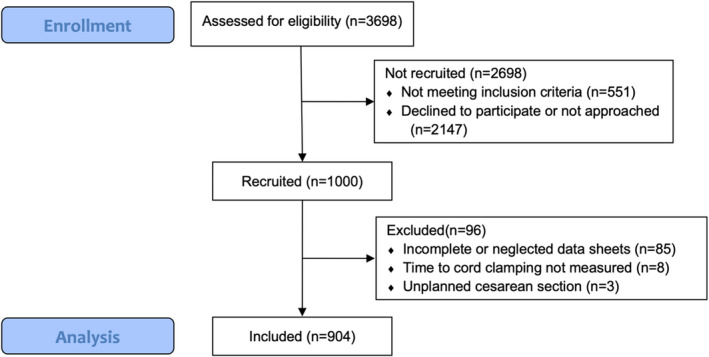
Flow diagram describing eligibility and inclusion of women and their newborn [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Maternal and neonatal characteristics
| Mean ± SD | N | |
|---|---|---|
| Maternal characteristics | ||
| Maternal age (y) | 30.3 ± 4.4 | 904 |
| Weight at first antenatal visit (kg) | 69.4 ± 13.9 | 644 |
| Body mass index (kg/m2) | 24.8 ± 4.6 | 640 |
| Umbilical artery pH | 7.25 ± 0.08 | 749 |
| Umbilical artery base deficit | 5.3 ± 3.4 | 729 |
| Time to umbilical cord clamping (min) | 6.4 ± 2.8 | 904 |
| Estimated postpartum blood loss volume (mL) | 405 ± 295 | 904 |
| Time of placenta delivery (min) | 12.7 ± 11.0 | 896 |
| Placental weight (g) | 613 ± 119 | 870 |
| Neonatal characteristics | ||
| Gestational age (wks) | 40.1 ± 1.3 | 902 |
| Birth weight (g) | 3604 ± 467 | 904 |
| Birth length (cm) | 50.7 ± 2.0 | 903 |
| Head circumference (cm) | 35.0 ± 1.4 | 899 |
| Apgar score at 1 min | 8.9 ± 0.7 | 904 |
| Apgar score at 5 min | 9.9 ± 0.4 | 904 |
| Apgar score at 10 min | 10.0 ± 0.2 | 904 |
| Age at metabolic screening (h) | 57.3 ± 11.9 | 885 |
| Transcutaneous bilirubin at metabolic screening (mg/dL) | 7.91 ± 3.90 | 866 |
Abbreviation: SD, standard deviation.
TABLE 2.
Prespecified maternal and neonatal outcome characteristics comparing umbilical cord clamping ≤3 min vs >3 min
| Time to umbilical cord clamping | Mean difference (95% confidence interval) | ||||
|---|---|---|---|---|---|
| ≤3 min | >3 min | ||||
| Mean ± SD | N | Mean ± SD | N | ||
| Maternal outcome | |||||
| Time to placental delivery (min) | 9.7 ± 11.9 | 62 | 12.9 ± 10.9 | 834 | 3.3 (0.4‐6.1) |
| Total estimated postpartum blood loss (mL) | 477 ± 326 | 63 | 399 ± 292 | 841 | −78 (−153‐−2) |
| Neonatal outcome | |||||
| Gestational age (weeks) | 40.1 ± 1.4 | 62 | 40.1 ± 1.2 | 840 | 0.0 (−0.3‐0.3) |
| Apgar score at 1 min | 8.0 ± 2.0 | 63 | 9.0 ± 0.5 | 841 | 1.0 (0.5‐1.5) |
| Apgar score at 5 min | 9.4 ± 1.2 | 63 | 10.0 ± 0.3 | 841 | 0.6 (0.1‐0.9) |
| Apgar score at 10 min | 9.7 ± 0.7 | 63 | 10.0 ± 0.1 | 841 | 0.3 (0.1‐0.5) |
| Umbilical artery pH | 7.23 ± 0.10 | 53 | 7.25 ± 0.08 | 695 | 0.02 (0.00‐0.04) |
| N (percent) | Total | N (percent) | Total | Odds ratio a (95% confidence interval) | |
|---|---|---|---|---|---|
| Phototherapy | 0 (0) | 63 | 6 (0.7) | 841 | NA† |
| Apgar score <7 at 5 min | 2 (3.2) | 63 | 0 (0) | 841 | NA‡ |
| Admission to neonatal ward | 7 (11.1) | 63 | 19 (2.3) | 841 | 0.2 (0.1‐0.5) |
| Respiratory distress | 4 (6.3) | 63 | 7 (0.8) | 841 | 0.1 (0.0‐0.4) |
| Infection | 1 (1.6) | 63 | 7 (0.8) | 841 | NA§ |
| Hypoglycemia | 2 (3.2) | 63 | 3 (0.4) | 841 | 0.1 (0.0‐0.7) |
| At high risk of hyperbilirubinemia requiring phototherapy | 14 (22.6) | 62 | 132 (16.4) | 805 | NA|| |
Note: † P > .99; ‡ P = .005; § P = .44; || P = .22.
Abbreviation: NA, Not applicable.
Group comparisons by Fisher exact test when odds ratio is NA.
3.2. Cord clamping time
The mean CC time was 6 minutes and 23 seconds and ranged from immediately at birth to 23 minutes and 31 seconds. CC was performed ≤3 minutes (180 seconds) in 63 infants (7.0%), ranged 0‐180 seconds, and mean ± SD was 123 ± 53 seconds.
3.3. Need for phototherapy
Phototherapy was initiated in six (0.7%) of the infants. CC time (mean ± SD) was not significantly different between those needing phototherapy (5.6 ± 2.2 minutes), and those not needing phototherapy (6.4 ± 2.8 minutes, P = .50). Transcutaneous bilirubin was measured in 866 (95.8%) newborns at the time of metabolic screening, with a median (min‐max) of 53.2 (47.1‐115.1) hours. No significant correlation was seen between bilirubin and time to CC (Spearman's rho −.014, P = .68). Transcutaneous bilirubin measured at the time of metabolic screening (mean ± SD) was similar between infants with CC ≤3 minutes (8.3 ± 4.1 mg/dL) and >3 minutes (7.9 ± 3.9 mg/dL), P = 0.46 (Figure 2A). CC time was compared among infants with low (n = 623), intermediate (n = 98), and high risk (n = 146) of needing phototherapy. In the multicomparison post hoc test, no significant differences in CC time were seen between risk groups (P = .29): CC time (mean ± SD) in the low‐risk group was 6.4 ± 2.8 minutes, in the intermediate‐risk group it was 6.0 ± 3.2 minutes, and in the high‐risk group it was 6.3 ± 2.9 minutes. For logistic regression, gestational age, age for metabolic screening, and primiparity were identified as independent variables and entered in the model. Due to so few newborns being subjected to phototherapy (n = 6), we could not construct a robust model and chose to enter “being at high risk of hyperbilirubinemia requiring phototherapy” as the dependent variable. The final model was robust, χ2 (df 4) = 42.1, P < .001, Cox and Snell R 2 = .05, and Nagelkerke R 2 = .08; for CC ≤3 minutes, B = 0.3, odds ratio (CI) = 1.4 (0.7 to 2.7), and P = .34.
FIGURE 2.
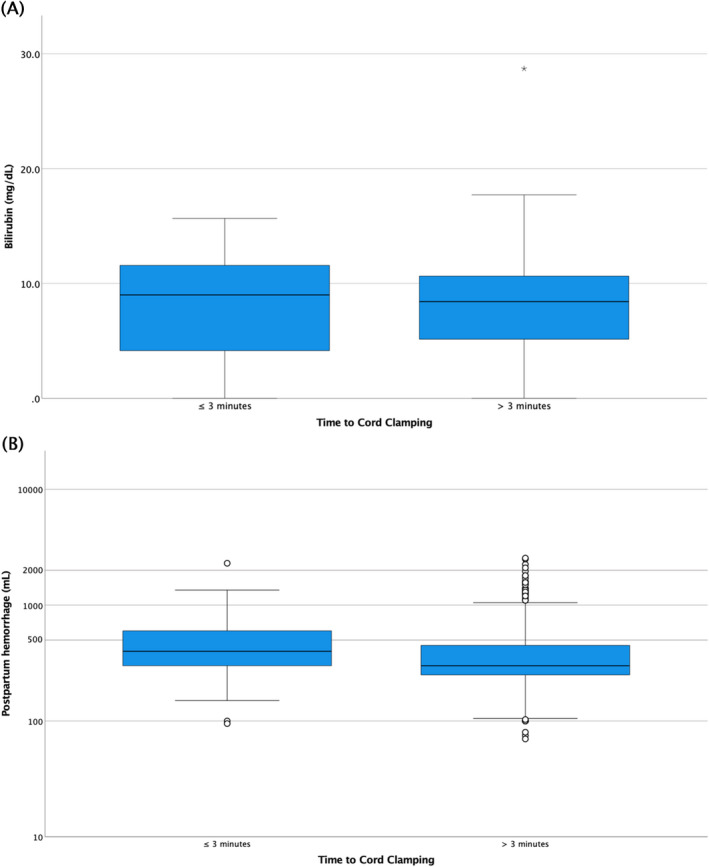
(A) Transcutaneous bilirubin measured at time for metabolic screening, comparing umbilical cord clamping ≤3 min vs >3 min. (B) Postpartum hemorrhage, comparing umbilical cord clamping ≤3 min vs >3 min [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Admission to the neonatal ward and neonatal short‐term outcome
As prespecified in the protocol, we divided newborns into groups with umbilical CC ≤3 minutes and >3 minutes. Admission to the neonatal ward (11.1% vs 2.3%, P = .001), respiratory distress (6.3% vs 0.8%, P = .005), hypoglycemia (3.2% vs 0.4%, P = .04), and Apgar <7 at 5 minutes of age (3.2% vs 0%, P = .005) were more common in the CC ≤3 minutes group.
As a post hoc analysis, we aimed to exclude the neonates presumed to be most hypoxic by excluding those with CC < 30 seconds and comparing infants with CC 30 seconds to ≤3 minutes to infants with CC >3 minutes (Table S1). The group with CC >3 minutes was still associated with significantly higher Apgar scores at 5 minutes, and fewer of the newborns clamped >3 minutes were admitted to the neonatal unit or presented with respiratory distress and/or with hypoglycemia.
3.5. Timing of umbilical CC and maternal postpartum blood loss
Total estimated postpartum blood loss was available for all women (Table 1). A negative significant correlation between postpartum blood loss and CC time, Spearman's rho −.115, P < .001, was found (Figure 2). The postpartum blood loss was significantly smaller in the group clamped >3 minutes compared with those with a CC time ≤3 minutes, mean difference 78 mL (95% CI 2‐153 mL) and the frequency of PPH (postpartum blood loss >500 mL) was twice as high in the group clamped ≤3 minutes, odds ratio 2.1 (95% CI 1.2 to 3.6) (Table 2 and Figure 2B).
For adjusted analysis, gestational age, placental weight, and delivery department were identified as independent variables entered in the logistic regression model; χ2 (df 3) = 32.1, P < .001, Cox and Snell R 2 = .04, and Nagelkerke R 2 = .06. For CC ≤3 minutes, B = 0.7, odds ratio (95% CI) = 2.0 (1.2 to 3.6), and P = .02.
3.6. Timing of administration of postdelivery oxytocin
Oxytocin was administered before CC in 722 (80%) deliveries and after CC in 167 (18%) deliveries. In 15 (2%), oxytocin was not administered at all or data were missing. The postpartum blood loss was (mean ± SD) 398 ± 301 mL if oxytocin was administered before CC and 408 ± 232 if administered after CC (P = .68). Transcutaneous bilirubin at metabolic screening was 134 ± 67 μmol/L if oxytocin was administered before and 138 ± 68 if administered after CC (P = .46). The administration of oxytocin before or after CC did not affect postpartum blood loss or neonatal morbidity (Table 3).
TABLE 3.
Comparisons between the timing of the administration of oxytocin (before/after umbilical cord clamping [CC]) on maternal postpartum hemorrhage and neonatal outcomes
| Oxytocin administration | P a | ||
|---|---|---|---|
|
Before CC N = 722 |
After CC (N = 167) |
||
| Maternal | |||
| Postpartum hemorrhage >500 mL | 135 (18.7%) | 32 (19.2%) | .91 |
| Severe postpartum hemorrhage >1000 mL | 28 (3.9%) | 6 (3.6%) | >.99 |
| Newborn | |||
| Apgar score <7 at 5 min | 1 (0.1%) | 1 (0.6%) | .34 |
| Admission to neonatal ward | 16 (2.2%) | 8 (4.8%) | .11 |
| Phototherapy treatment | 5 (0.7%) | 1 (0.6%) | >.99 |
| Respiratory distress | 7 (1.0%) | 4 (2.4%) | .13 |
| Infection | 6 (0.8%) | 2 (1.2%) | .65 |
| Hypoglycemia | 3 (0.4%) | 1 (0.6%) | .67 |
Note: Respiratory distress, infection, and hypoglycemia as reported in the study protocol.
Abbreviation: CC, umbilical cord clamping.
Group comparisons by Fisher exact test when odds ratio is NA.
4. DISCUSSION
This large prospective observational study of 904 mother‐infant dyads shows an absence of correlation between delayed CC beyond 3 minutes and negative short‐term neonatal outcomes, including increased risk of phototherapy, in term and late‐preterm infants. Furthermore, a negative correlation between CC time and postpartum blood loss was observed. The administration of oxytocin before or after CC was not associated with any differences in postpartum blood loss or neonatal morbidity. The actual time of CC was much longer than recommended in Sweden. 19
The absence of correlation between CC time and risk of phototherapy is a particularly important finding since influential stakeholders, such as the American College of Obstetricians and Gynecologists, remark that jaundice requiring phototherapy is less common among newborns with early CC. 13 In addition, a recent meta‐analysis on CC management raises the concern that placental transfusion might be associated with a greater risk of hyperbilirubinemia requiring phototherapy. 20 Our results contradict these concerns. In addition, the potential risks of need for phototherapy after delayed CC refer to a 2013 Cochrane review, where some results are questionable, as studies in which decisions to initiate phototherapy were based on clinical evaluation, and not bilirubin levels, were included. 12
Furthermore, our findings are similar to large randomized controlled trials which reported no relation between the need of phototherapy and CC time. 21 , 22 , 23 Chen (n = 720) reported similar neonatal transcutaneous bilirubin values at 72 hours of age and found no significant difference in need for phototherapy among groups (eight groups; CC within 15 seconds, CC by 30, 60, 90, 120, 150, or 180 seconds, or when umbilical cord pulsations ceased). 21 Rana (n = 524) did not find any differences in transcutaneous bilirubin at discharge or parent‐reported jaundice or phototherapy when comparing infants with CC time within 1 minute to after 3 minutes. 22 Mohammad (n = 128) analyzed bilirubin levels 12 hours and 3 days after birth and did not observe differences between early (<30 seconds) and delayed CC (90 s). 23 Our findings suggest that concerns around the need for phototherapy in relation to delayed CC can be abandoned.
Another important finding in this study is the correlation between postpartum blood loss and CC time <3 minutes. These findings are concordant with the results from a Cochrane review involving 1257 women showing that placental cord drainage reduced the length of the third stage of labor and reduced blood loss by an average of 77 mL. 24 However, with an observational design, increased postpartum blood loss at lower CC times may also mirror a propensity to cut the umbilical cord earlier, when the mother is bleeding. Yet, our findings suggest that increased blood loss in conjunction with delayed CC may be of less concern.
Cord clamping <3 minutes was associated with lower Apgar scores at 5 and 10 minutes compared with CC >3 minutes. This finding aligns with previous studies showing that delayed CC >3 minutes is associated with short‐term outcomes such as improved oxygen levels immediate after birth. 25 , 26
The strengths of the study include the prospective design, and the number of mothers and newborns included and that the results are congruent with recent studies. Of particular interest is the timing of CC, with a mean of 6 minutes and 23 seconds. This is much longer than times previously reported in most research and approaches the timing for the end of third‐stage labor and the delivery of the placenta; the end of third stage has been described as the time to cut the cord in a few studies from Sweden, Canada and The Netherlands. 16 , 27 , 28 This study shows that longer times to CC is common in Swedish delivery departments.
A limitation to this study is the relatively small number of infants in the group with a CC ≤3 minutes. There is a risk of selection bias, as we could only include approximately one third of the eligible dyads and midwives prone to delayed CC might have been more active in recruiting. The study was performed in two highly equipped settings, with deliveries handled by licensed midwives, which might affect global generalizability. Assessing postpartum blood loss is very difficult and subject to several sources of error. Although the assessment of postpartum blood loss was the same throughout the study, systematic errors can not be discounted.
4.1. Conclusions
Delaying CC beyond 3 minutes was not associated with any negative consequences for mother or child in this study of 904 mother‐infant dyads. This further strengthens the recommendation to delay CC >3 minutes to give the infant short‐term benefits such as improved oxygen levels immediately after birth 25 , 26 and positive long‐term benefits such as less iron deficiency, improved brain myelin content, neurodevelopment, and reduction of infant anemia in low‐resource settings. 3 , 4 , 5 , 6 , 7 , 8 , 9 Furthermore, the results suggest that the concerns about increased risk of phototherapy in conjunction with delayed CC should be abandoned. However, longer CC time than evaluated in this study, for example extending CC time beyond placental delivery, could theoretically lead to reverse blood transfusion back to the placenta 29 and is not recommended. Although CC time as long as 6 minutes could be considered as safe, further research will be needed to decide the optimal timing for mother and child.
CONFLICT OF INTEREST
None of the authors have financial involvement that could represent potential conflict of interest.
ETHICAL APPROVAL
The study was approved by the Regional ethical review board at Lund University (2016/1052).
Supporting information
Table S1
ACKNOWLEDGMENT
Regional Scientific Council of Halland.
Winkler A, Isacson M, Gustafsson A, Svedenkrans J, Andersson O. Cord clamping beyond 3 minutes: Neonatal short‐term outcomes and maternal postpartum hemorrhage. Birth. 2022;49:783‐791. doi: 10.1111/birt.12645
This is an observational cohort study conducted at the two delivery departments at the Hospital of Halland, in the cities of Varberg and Halmstad, Sweden, during the period April 28, 2017 to March 18, 2018. Three of the authors are affiliated to Lund University or Karolinska Institute, Stockholm, respectively.
The study was partly presented as a poster at the 2018 PAS Annual meeting, May 8, 2018, Toronto, Canada.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Yao AC, Moinian M, Lind J. Distribution of blood between infant and placenta after birth. Lancet. 1969;2(7626):871‐873. doi: 10.1016/s0140-6736(69)92328-9 [DOI] [PubMed] [Google Scholar]
- 2. Farrar D, Airey R, Law GR, Tuffnell D, Cattle B, Duley L. Measuring placental transfusion for term births: weighing babies with cord intact. BJOG. 2011;118(1):70‐75. doi: 10.1111/j.1471-0528.2010.02781.x [DOI] [PubMed] [Google Scholar]
- 3. Andersson O, Hellstrom‐Westas L, Andersson D, Domellof M. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: a randomised controlled trial. BMJ. 2011;343:d7157. doi: 10.1136/bmj.d7157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaparro CM, Neufeld LM, Tena Alavez G, Eguia‐Liz Cedillo R, Dewey KG. Effect of timing of umbilical cord clamping on iron status in Mexican infants: a randomised controlled trial. Lancet. 2006;367(9527):1997‐2004. doi: 10.1016/S0140-6736(06)68889-2 [DOI] [PubMed] [Google Scholar]
- 5. Kc A, Rana N, Malqvist M, Jarawka Ranneberg L, Subedi K, Andersson O. Effects of delayed umbilical cord clamping vs early clamping on anemia in infants at 8 and 12 months: a randomized clinical trial. JAMA Pediatr. 2017;171(3):264‐270. doi: 10.1001/jamapediatrics.2016.3971 [DOI] [PubMed] [Google Scholar]
- 6. Rana N, Kc A, Malqvist M, Subedi K, Andersson O. Effect of delayed cord clamping of term babies on neurodevelopment at 12 months: a randomized controlled trial. Neonatology. 2019;115(1):36‐42. doi: 10.1159/000491994 [DOI] [PubMed] [Google Scholar]
- 7. Andersson O, Lindquist B, Lindgren M, Stjernqvist K, Domellof M, Hellstrom‐Westas L. Effect of delayed cord clamping on neurodevelopment at 4 years of age: a randomized clinical trial. JAMA Pediatr. 2015;169(7):631‐638. doi: 10.1001/jamapediatrics.2015.0358 [DOI] [PubMed] [Google Scholar]
- 8. Mercer JS, Erickson‐Owens DA, Deoni SCL, et al. Effects of delayed cord clamping on 4‐month ferritin levels, brain myelin content, and neurodevelopment: a randomized controlled trial. J Pediatr. 2018;203:266‐272.e2. doi: 10.1016/j.jpeds.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mercer JS, Erickson‐Owens DA, Deoni SCL, et al. The effects of delayed cord clamping on 12‐month brain myelin content and neurodevelopment: a randomized controlled trial. Am J of Perinatol. 2022;39(01):37‐44. doi: 10.1055/s-0040-1714258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fogarty M, Osborn DA, Askie L, et al. Delayed vs early umbilical cord clamping for preterm infants: a systematic review and meta‐analysis. Am J Obstet Gynecol. 2018;218(1):1‐18. doi: 10.1016/j.ajog.2017.10.231 [DOI] [PubMed] [Google Scholar]
- 11. Rabe H, Gyte GM, Diaz‐Rossello JL, Duley L. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. 2019;9:CD003248. doi: 10.1002/14651858.CD003248.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2013;2013(7):CD004074. doi: 10.1002/14651858.CD004074.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Delayed umbilical cord clamping after birth: ACOG Committee Opinion, number 814. Obstet Gynecol. 2020;136(6):e100‐e106. doi: 10.1097/aog.0000000000004167 [DOI] [PubMed] [Google Scholar]
- 14. Prendiville WJ, Harding JE, Elbourne DR, Stirrat GM. The Bristol third stage trial: active versus physiological management of third stage of labour. BMJ. 1988;297(6659):1295‐1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vain NE, Satragno DS, Gordillo JE, et al. Postpartum use of oxytocin and volume of placental transfusion: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2020;105(1):14‐17. doi: 10.1136/archdischild-2018-316649 [DOI] [PubMed] [Google Scholar]
- 16. Isacson M, Thies‐Lagergren L, Oras P, Hellström‐Westas L, Anderssson O. Umbilical cord clamping and management of the third stage of labor: A telephone‐survey describing Swedish midwives’ clinical practice. European Journal of Midwifery. 2022;6:1‐7. doi: 10.18332/ejm/145697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour‐specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near‐term newborns. Pediatrics. 1999;103(1):6‐14. doi: 10.1542/peds.103.1.6 [DOI] [PubMed] [Google Scholar]
- 18. Varvarigou A, Fouzas S, Skylogianni E, Mantagou L, Bougioukou D, Mantagos S. Transcutaneous bilirubin nomogram for prediction of significant neonatal hyperbilirubinemia. Pediatrics. 2009;124(4):1052‐1059. doi: 10.1542/peds.2008-2322 [DOI] [PubMed] [Google Scholar]
- 19. Wiklund I, Nordström L, Norman M. Vårdprogram för avnavling av nyfödda barn [Care program for umbilical cord clamping of newborn children]. Lakartidningen. 2008;105(45):3208‐3210. [PubMed] [Google Scholar]
- 20. Gomersall J, Berber S, Middleton P, et al. Umbilical cord management at term and late preterm birth: a meta‐analysis. Pediatrics. 2021;147(3):e2020015404. doi: 10.1542/peds.2020-015404 [DOI] [PubMed] [Google Scholar]
- 21. Chen X, Li X, Chang Y, Li W, Cui H. Effect and safety of timing of cord clamping on neonatal hematocrit values and clinical outcomes in term infants: a randomized controlled trial. J Perinatol. 2018;38(3):251‐257. doi: 10.1038/s41372-017-0001-y [DOI] [PubMed] [Google Scholar]
- 22. Rana N, Ranneberg LJ, Malqvist M, Kc A, Andersson O. Delayed cord clamping was not associated with an increased risk of hyperbilirubinaemia on the day of birth or jaundice in the first 4 weeks. Acta Paediatr. 2020;109(1):71‐77. doi: 10.1111/apa.14913 [DOI] [PubMed] [Google Scholar]
- 23. Mohammad K, Tailakh S, Fram K, Creedy D. Effects of early umbilical cord clamping versus delayed clamping on maternal and neonatal outcomes: a Jordanian study. J Matern Fetal Neonatal Med. 2021;34(2):231‐237. doi: 10.1080/14767058.2019.1602603 [DOI] [PubMed] [Google Scholar]
- 24. Soltani H, Poulose TA, Hutchon DR. Placental cord drainage after vaginal delivery as part of the management of the third stage of labour. Cochrane Database Syst Rev. 2011;2011(9):CD004665. doi: 10.1002/14651858.CD004665.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kc A, Singhal N, Gautam J, Rana N, Andersson O. Effect of early versus delayed cord clamping in neonate on heart rate, breathing and oxygen saturation during first 10 minutes of birth ‐ randomized clinical trial. Matern Health Neonatol Perinatol. 2019;5:7. doi: 10.1186/s40748-019-0103-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Katheria AC, Brown MK, Faksh A, et al. Delayed cord clamping in newborns born at term at risk for resuscitation: a feasibility randomized clinical trial. J Pediatr. 2017;187:313‐317.e1. doi: 10.1016/j.jpeds.2017.04.033 [DOI] [PubMed] [Google Scholar]
- 27. Fulton C, Stoll K, Thordarson D. Bedside resuscitation of newborns with an intact umbilical cord: experiences of midwives from British Columbia. Midwifery. 2016;34:42‐46. doi: 10.1016/j.midw.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 28. Boere I, Smit M, Roest AAW, Lopriore E, Van Lith JMM, Te Pas AB. Current practice of cord clamping in The Netherlands: a questionnaire study. Neonatology. 2015;107(1):50‐55. doi: 10.1159/000365836 [DOI] [PubMed] [Google Scholar]
- 29. Svedenkrans J, Aquilano G, Pettersson K. A case of severe infant‐to‐placenta hemorrhage in association with prolonged delayed cord clamping. Am J Case Rep. 2020;21:e925116. doi: 10.12659/ajcr.925116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


