FIGURE 1.
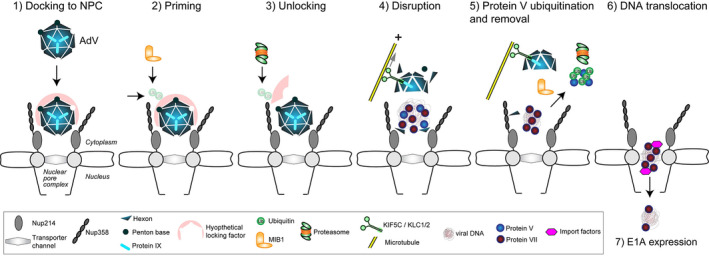
An inclusive model for adenovirus disassembly at the NPC and nuclear import of viral DNA. The HAdV‐C5 capsid with pseudo T = 25 icosahedral symmetries effectively protects and efficiently delivers its DNA cargo into host cells. During infectious cell entry, virions are dismantled in a coordinated and well‐controlled manner (Greber et al., 1994). At the plasma membrane, the virion sheds the fibers and some of the penton base proteins and, subsequently, also the internal stabilizing proteins IIIa and VIII (Greber et al., 1993; Nakano et al., 2000). It exposes the membrane lytic protein VI (Burckhardt et al., 2011; Wodrich et al., 2010), and upon rupture of the endosomal membrane, releases the protein VI, as well as a small fraction of the internal DNA decondensing protein V (Burckhardt et al., 2011; Puntener et al., 2011). The leaky virion attaches to microtubule motors and traffics toward the cell nucleus (Scherer et al., 2020; Wang et al., 2018), where it detaches from the microtubules and binds to the NPC protein Nup214 (Wang et al., 2017) (1). This binding occurs through the major capsid protein hexon (Cassany et al., 2015; Trotman et al., 2001). A locking factor, or the lack of critical ubiquitination providing a positive signal for dismantling, precludes the NPC‐docked virion from disassembly (Bauer et al., 2021). The E3 ubiquitin ligase MIB1 primes the virion for disassembly at the NPC by recruiting the disassembly machinery (2), or targeting the locking factor for proteasomal degradation (3). The disruption of the capsid is mediated by the conventional kinesin KIF5C and its light chain KLC1/2 pulling on microtubules near the NPC against the holding force of the nucleus (Joseph & Dasso, 2008; Strunze et al., 2011) (4). The TPR domain of KLC1/2 attaches the motor complex to the facet‐stabilizing protein IX (de Vrij et al., 2011; Strunze et al., 2011). Kinesin activation might be mediated by binding of Nup358 to the stalk of KIF5C, as shown by an unrelated in vitro study demonstrating allosteric activation of the ATPase activity (Cho et al., 2009). When the capsid is sufficiently disrupted, MIB1‐mediated ubiquitination of protein V leads to the detachment of V from the capsid and the viral DNA (5). This reaction renders the viral DNA in complex with the condensing protein VII a nuclear import substrate recognized by nuclear import factors (6). Remarkably, insufficient ubiquitination of protein V increases the misdelivery of viral DNA to the cytoplasm, instead of the nucleus, as shown by the all lysine to arginine HAdV‐C5 mutant (Bauer et al., 2021)
