Fig. 1.
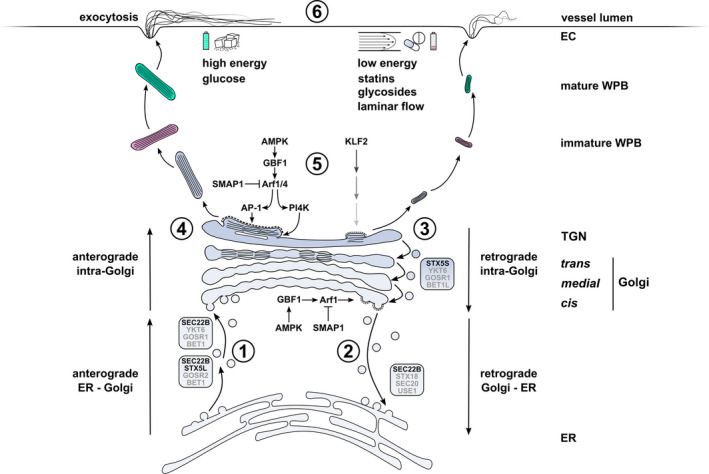
Regulatory mechanisms of WPB size and VWF secretion in the early secretory pathway of endothelial cells. Anterograde ER to Golgi trafficking of VWF via COPII vesicles is facilitated by SEC22B‐ and STX5‐ (STX5L: long isoform) containing SNARE complexes that mediate fusion at the Golgi. (2) Retrograde Golgi to ER membrane retrieval, which promotes cisternal maturation, anterograde transport of VWF through the Golgi and TGN exit is coordinated by active Arf1, which in turn recruits coatomer to COPI vesicles as they bud from the cis‐Golgi. Arf GAP SMAP1 deactivates Arf1 while Arf GEF GBF1 activates Arf1. GBF1 activity is modulated by the energy level‐dependent kinase AMPK. (3) Retrograde intra‐Golgi traffic via STX5‐containing SNARE complex (STX5S: short isoform). (4) Progression of VWF quanta through the Golgi stacks. The number of VWF quanta positioned directly next to one another at the TGN at the time of vesicle budding determines the length of the emerging WPB. (5) Arf1 and Arf4, regulated by SMAP1 and GBF1/AMPK, promote TGN exit by recruitment of the cargo adapter AP‐1 and clathrin to budding WPBs and via PI4K‐induced lipid modifications. Upregulation of the mechanosensitive transcription factor KLF2 promotes formation of smaller WPBs through a yet undefined mechanism that may involve Golgi fragmentation. (6) Dependent on type and strength of stimulus, mature WPBs are prioritized for exocytosis based on their size. Long WPBs release more VWF and produce longer VWF strings with increased platelet adhesive capacity, but require stronger stimulus. Environmental cues that are associated with long (left) and short (right) WPBs are indicated. Greyed out SNAREs (BET1, BET1L, GOSR1/2, SEC20, USE1 and YKT6) have been inferred from SNARE complexes in these pathways in other mammalian cells. Abbreviations: EC, endothelial cell; ER, endoplasmic reticulum; TGN, trans‐Golgi network; WPB, Weibel‐Palade body.
