FIGURE 2.
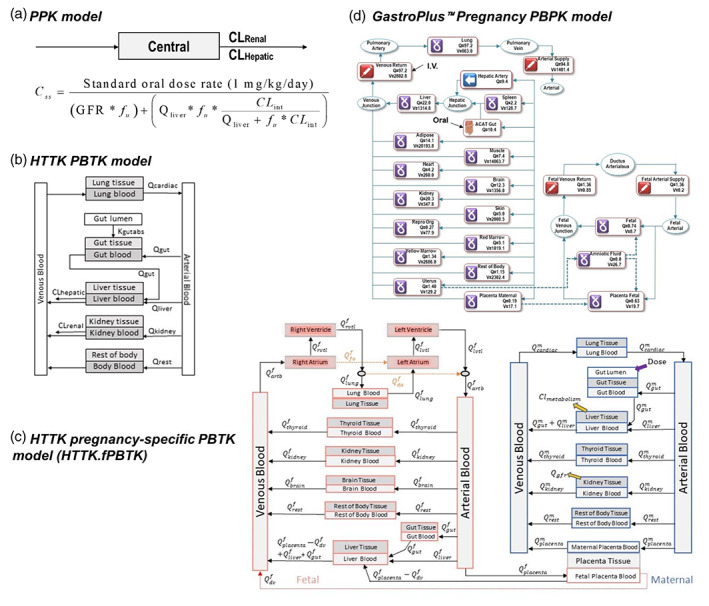
Structures of pharmacokinetic (PK) models used for in vivo to in vitro extrapolation analyses. (a) The population‐based pharmacokinetic model is a one‐compartment, open‐source population‐based PK model. (b) The httk.PBTK model is a generalized PBTK model provided in the Environmental Protection Agency (EPA) httk R package. (c) The httk.fPBTK model is a human pregnancy specific PBTK model provided in the EPA httk R package. Adapted from Sfeir et al. (2020). (d) A human pregnancy physiologically based pharmacokinetic model included in GastroPlus software. Expressions such as “Qliver”, “Qkidney”, represent the blood flow rate in liters/hr (L/h) into and through that tissue. CLHepatic, hepatic clearance (L/h); CLint, intrinsic hepatic clearance (L/h); CLRenal, renal clearance (L/h); GFR, glomerular filtration rate (L/h); PBTK, physiologically based toxicokinetic; Qf, fetal tissue blood flow; Qm, maternal tissue blood flow; V, tissue volume.
