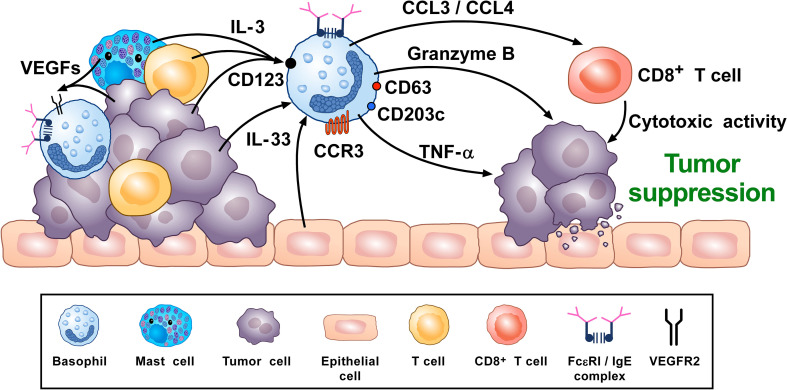
Figure 2.
Theoretical representation of how basophils can promote tumor suppression. Basophils have been located in the immune infiltrate of several human (80, 102, 261, 262) and experimental tumors (80, 97, 102, 276). Vascular endothelial growth factors released by cancer cells and immune cells in tumor microenvironment (TME) (e.g., mast cells, macrophages) (118, 120–123) can favor basophil infiltration in TME through the engagement of VEGFR2 on these cells (120). IL-3, produced by intratumoral lymphocytes, mast cells and cancer cells (17, 82, 281), is the most important growth and activating factor for human and mouse basophils, through the engagement of the IL-3 receptor (IL-3Rα/CD123) (17). CCL3/CCL4 secreted by intratumoral basophils induces CD8+ T cell recruitment in TME, promoting melanoma rejection in mice (270). IL-33, a pleiotropic cytokine produced by epithelial and tumor cells (282), plays a central role in tumorigenesis (282). IL-33 upregulates granzyme B mRNA and the surface expression of CD63, suggesting functional and phenotypic basophil activation. IL-33-activated mouse basophils induce melanoma cell death in vitro (67). Mouse (47, 86) and, under specific circumstances, human basophils (88, 89) release TNF-α. Human and mouse basophils release granzyme B (66, 67). Both TNF-α and granzyme B exert cytotoxic effects on tumor cells (68, 69). Activated signature (CD123, CCR3, CD63, CD203c gene expression) in tumor resident basophils is associated with improved outcome in ovarian cancer patients (248, 261). Topical exposure to a DNA-damaging carcinogen promotes tumor-protective IgE response through skin infiltrating basophils (276). Taken together, these results suggest that, in certain experimental and clinical conditions, basophils and their mediators may play an anti-tumorigenic role.