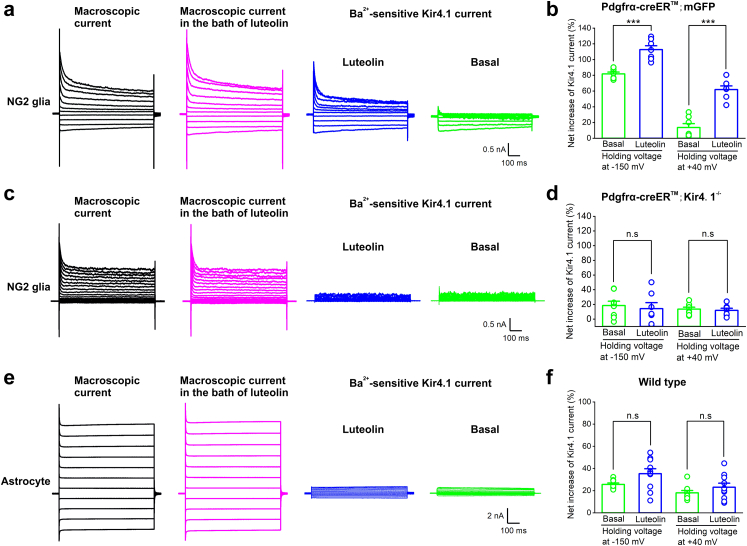
Fig. 4.
Luteolin specifically augments Kir4.1 channel currents in NG2 glia. (a) Representative traces show macroscopic current (in black), luteolin-induced macroscopic current (in magenta) and Ba2+-sensitive Kir4.1 currents after luteolin application (in blue) in NG2 glia from Pdgfrα-creER™;mGFP mice. (b) Bar graph summary showing the percentage of augmentation in Ba2+-sensitive Kir4.1 currents between control (basal) and luteolin application when the cell voltage was held at −150 mV and +40 mV, respectively. The error bars represent s.e.m. ∗∗∗p < 0.001, two-tailed unpaired t-test. n = 7 cells recorded for each group. (c) Representative traces show macroscopic current (in black), luteolin-induced macroscopic current (in magenta) and Ba2+-sensitive Kir4.1 currents after luteolin application (in blue) in NG2 glia from Pdgfrα-creER™; Kir4.1−/− mice. Note that luteolin did not induce the augmentation of Kir4.1 current in NG2 glia in Kir4.1 cKO mice. (d) Bar graph summary showing the percentage of augmentation in Ba2+-sensitive Kir4.1 currents between control (basal) and luteolin application when the cell voltage was held at −150 mV and +40 mV, respectively. The error bars represent s.e.m. n.s indicates not significant, two-tailed unpaired t-test. n = 8 and 7 cells for control and luteolin group, respectively. (e) Representative traces show macroscopic current (in grey), luteolin-induced macroscopic current (in magenta) and Ba2+-sensitive Kir4.1 current after luteolin application (in blue) in astrocytes from wild type mice. (f) Bar graph summary showing the percentage of augmentation in Ba2+-sensitive Kir4.1 currents between control (basal) and luteolin application when the cell voltage was held at −150 mV and +40 mV, respectively. The error bars represent s.e.m. n.s indicates not significant, two-tailed unpaired t-test. n = 9 and 10 cells for control and luteolin group, respectively.