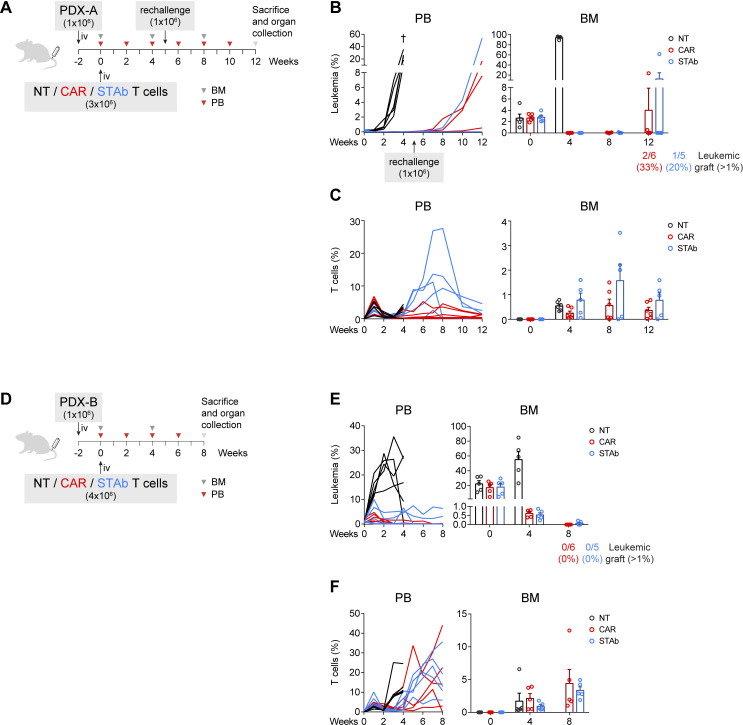
Figure 4.
CD1a-STAb and CD1a-CAR T cells are effective in eliminating primary coT-ALL in long-term in vivo models. (A, D) Experimental design of in vivo cytotoxicity in NSG mice receiving intravenous coT-ALL patient-derived xenograft (PDX-A in a and PDX-B in D) cells (1×106) followed by NT, CD1a-CAR or CD1a-STAb T cells 2 weeks later (3×106 in a and 4×106 in D). In (A), disease-free mice were rechallenged with further 1×106 PDX-A cells on week 5. (B, C) Percentage of leukemic (B) and T cells (C) in PB and BM at the indicated time points in the PDX-A model. (E, F) Percentage of leukemic (E) and T cells (F) in PB and BM at the indicated time points in the PDX-B model. Numbers of mice with leukemic graft at endpoint, determined as >1% blasts, are indicated. Leukemic blasts were identified by flow cytometry as HLA-ABC+CD45+CD1a+CD3– and CD34+ (PDX-A) or CD38+ (PDX-B), and T cells as HLA-ABC+CD45+CD1a–CD3+. BM plots from B, C) and E, F) show mean±SEM of at least 5 mice per group. ALL, acute lymphoblastic leukemia; BM, bone marrow; PB, peripheral blood; NT, non-transduced; CAR, chimeric antigen receptor; STAb, secreting T cell-redirecting antibodies.