Abstract
Background
Some studies have reported that influenza vaccination is associated with lower risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and/or coronavirus disease 2019 (COVID-19) morbidity and mortality. This study aims to estimate effectiveness of influenza vaccination, using Abbott’s quadrivalent Influvac Tetra vaccine, against SARS-CoV-2 infection and against severe COVID-19.
Methods
This matched, test-negative, case-control study was implemented on a population of 30,774 healthcare workers (HCWs) in Qatar during the 2020 annual influenza vaccination campaign, September 17, 2020-December 31, 2020, before introduction of COVID-19 vaccination.
Results
Of 30,774 HCWs, 576 with PCR-positive tests and 10,033 with exclusively PCR-negative tests were eligible for inclusion in the study. Matching by sex, age, nationality, reason for PCR testing, and PCR test date yielded 518 cases matched to 2058 controls. Median duration between influenza vaccination and the PCR test was 43 days (IQR, 29–62). Estimated effectiveness of influenza vaccination against SARS-CoV-2 infection> 14 days after receiving the vaccine was 29.7% (95% CI: 5.5–47.7%). Estimated effectiveness of influenza vaccination against severe, critical, or fatal COVID-19 was 88.9% (95% CI: 4.1–98.7%). Sensitivity analyses confirmed the main analysis results.
Conclusions
Recent influenza vaccination is associated with a significant reduction in the risk of SARS-CoV-2 infection and COVID-19 severity.
Keywords: Influenza, COVID-19, Vaccine, Case-control, Immunity, Epidemiology
Introduction
Influenza vaccination protects against influenza infection and reduces morbidity and mortality of seasonal influenza [1]. This vaccination is strongly recommended for high-risk groups such as elderly persons and healthcare workers (HCWs) [2]. A number of studies have reported that influenza vaccination is also associated with lower risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and/or coronavirus disease 2019 (COVID-19) morbidity and mortality [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]. However, reported apparent protection against SARS-CoV-2 infection and severe COVID-19 could have arisen due to bias and may not have reflected a genuine biological effect. In particular, this protection may have been due to the healthy user effect [14], whereby health-aware persons are more likely to receive an influenza vaccine, and simultaneously, to practice health behaviors that reduce their risk of acquiring the infection [3], [5].
Against this background, we assessed effectiveness of vaccination with Abbott’s quadrivalent Influvac Tetra vaccine against SARS-CoV-2 infection, as documented using polymerase chain reaction (PCR) testing. Effectiveness of influenza vaccination was also assessed against infection progression to severe (acute-care hospitalization) [15], critical (intensive-care-unit hospitalization) [15], or fatal [16] COVID-19 disease, per World Health Organization definitions [15], [16]. Both of these effectiveness measures were assessed among HCWs in Qatar during the 2020 annual influenza vaccination campaign, and before introduction of COVID-19 vaccination. Since variation in health behaviors among HCWs is presumably less than that among the general population, conducting this study among HCWs allowed us to minimize the influence of the healthy user effect on effectiveness estimates.
Materials and methods
Study design
This study was retrospective and conducted among HCWs at Hamad Medical Corporation, the principal provider of public healthcare services in Qatar and the nationally designated entity for COVID-19-related healthcare needs. The study analyzed the centralized, national, federated databases for COVID-19 laboratory testing, vaccination, hospitalization, and death, retrieved from the integrated nationwide digital-health information platform. These databases include all SARS-CoV-2-related data and associated demographic information, with no missing information, since the pandemic onset, documenting all PCR tests. Influenza vaccination data were retrieved from Hamad Medical Corporation’s influenza vaccination database. Further descriptions of these databases have been reported previously [17], [18], [19], [20], [21], [22], [23].
SARS-CoV-2 testing in the healthcare system in Qatar was done at a mass scale, and mostly for routine reasons [18], [24]. Most infections were diagnosed due to routine testing rather than because of symptoms [18]. Every PCR test conducted in Qatar, regardless of location or setting, was tracked nationally and was classified based on symptoms present at the time of testing and the reason for testing (clinical symptoms, contact tracing, surveys or random testing campaigns, individual requests, routine healthcare testing, pre-travel, screening at ports of entry, or other). All PCR testing done during the present study was factored in the analyses of this study. Qatar has unusually young, diverse demographics, in that only 9% of its residents are ≥ 50 years of age, and 89% are expatriates from over 150 countries [17]. This diversity also characterizes the HCW population.
Effectiveness of influenza vaccination against SARS-CoV-2 infection was assessed using the test-negative, case-control study design, a preferred design for assessing vaccine effectiveness against influenza [25], [26]. This design was also validated in Qatar’s population and has been repeatedly applied in previous studies in this country to assess COVID-19 vaccine effectiveness [18], [20], [21], [24], [27], [28], [29].
SARS-CoV-2 PCR testing and vaccination records were retrieved for all HCWs at HMC. Any HCW with a SARS-CoV-2 PCR test performed between September 17, 2020 and December 31, 2020 was eligible for inclusion in the study. This duration coincided with the national annual influenza vaccination campaign that typically starts in September or October, and continues for several months thereafter. Influenza vaccination is offered free of charge to all citizens and residents and is offered in both public and private healthcare institutions. During the 2020 influenza vaccination campaign, only Abbott’s quadrivalent Influvac Tetra vaccine was used in the national influenza vaccination campaign. Individuals who did not receive this influenza vaccine are unlikely to have received any other influenza vaccine.
The study was concluded at the start of the mass COVID-19 vaccination campaign to ensure that the estimated effectiveness of the influenza vaccine does not factor any protective effect for SARS-CoV-2 vaccination. During the study, SARS-CoV-2 incidence was due to the original SARS-CoV-2 virus, before introduction of SARS-CoV-2 variants of concern [30], [31], [32], [33], [34]. SARS-CoV-2 incidence was also relatively low during the study with no wave materializing during this time [33], [35].
To estimate effectiveness against SARS-CoV-2 infection, we exact-matched cases (HCWs with PCR-positive tests) and controls (HCWs with PCR-negative tests) identified during the study in a 1:5 ratio by sex, 10-year age groups, 10-nationality groups, reason for PCR testing, and bi-weekly PCR test date, to control for known differences in SARS-CoV-2 exposure risk in Qatar [17], [35], [36], [37], [38]. Matching by these factors was shown previously in studies of different epidemiologic designs to provide adequate control of differences in the risk of exposure to SARS-CoV-2 infection in Qatar [18], [21], [23], [29], [39].
Only the first PCR-positive test for cases and the first PCR-negative test for controls during the study were included in the present analysis. Controls included individuals with no record of a PCR-positive test during the study period. HCWs with a PCR test within 14 days after receiving the influenza vaccine and those who received COVID-19 vaccination were excluded. These inclusion and exclusion criteria were implemented to allow adequate time for build-up of immunity after vaccination [20], and to minimize different types of potential bias, as informed by earlier analyses on the same population [18], [29]. Every control that met the inclusion criteria and that could be matched to a case was included in the analysis.
Classification of COVID-19 case severity [15], criticality [15], and fatality [16] followed World Health Organization guidelines, and assessments were made by trained medical personnel using individual chart reviews, as part of a national protocol applied to every hospitalized COVID-19 patient. Details of COVID-19 severity, criticality, and fatality classifications are found in Supplementary Section 1.
Every hospitalized COVID-19 patient underwent infection severity assessment every three days until discharge or death. We classified individuals who progressed to severe, critical, or fatal COVID-19 between the time of the PCR-positive test and the end of the study based on their worst disease outcome, starting with death [16], followed by critical disease [15], and then severe disease [15]. Individuals with a PCR-documented infection, but no COVID-19 hospitalization record, were assumed to have a SARS-CoV-2 infection that did not progress to severe, critical, or fatal COVID-19, that is asymptomatic, mild or moderate COVID-19.
Laboratory methods
Details of laboratory methods for real-time reverse-transcription PCR (RT-qPCR) testing are found in Supplementary Section 2. All PCR testing was conducted at the Hamad Medical Corporation Central Laboratory or at Sidra Medicine Laboratory, following standardized protocols.
Statistical analysis
Baseline characteristics of cases and controls were reported using descriptive statistics. Groups were compared using standardized mean differences (SMDs), with SMD below or close to 0.1 indicating adequate matching [40]. Conditional logistic regression factoring the matched design was performed to compare odds of influenza vaccination in cases versus controls. This analytical approach, including matching by bi-weekly PCR test date, minimizes potential bias due to variation in epidemic phase [25], [41] and roll-out of vaccination during the study [25], [41]. Interactions were not considered. The resulting estimates for the adjusted odds ratio and 95% confidence interval (CI) were then used to estimate influenza vaccination effectiveness and corresponding 95% CI using the equation [25], [26]:
Effectiveness was estimated against SARS-CoV-2 infection, whether symptomatic or asymptomatic, and against any severe, critical, or fatal COVID-19. Sensitivity analyses were conducted to investigate the impact of adjusting for prior SARS-CoV-2 infection in the conditional logistic regression, and of modifying the study inclusion and exclusion criteria on the estimate of influenza vaccination effectiveness. The latter was informed by an established analysis plan to investigate sources of potential bias [18], [29], and encompassed exclusion of individuals with prior SARS-CoV-2 infection, exclusion of travel-related PCR testing, or inclusion of all PCR-positive and PCR-negative tests for cases and controls. Statistical analyses were conducted using STATA/SE version 17.0 (Stata Corporation, College Station, TX, USA).
Oversight
Hamad Medical Corporation and Weill Cornell Medicine-Qatar Institutional Review Boards approved this retrospective study with a waiver of informed consent. The research was performed in accordance with relevant guidelines and regulations and in accordance with the Declaration of Helsinki [42]. The study was reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The STROBE checklist can be found in Supplementary Table 1.
Results
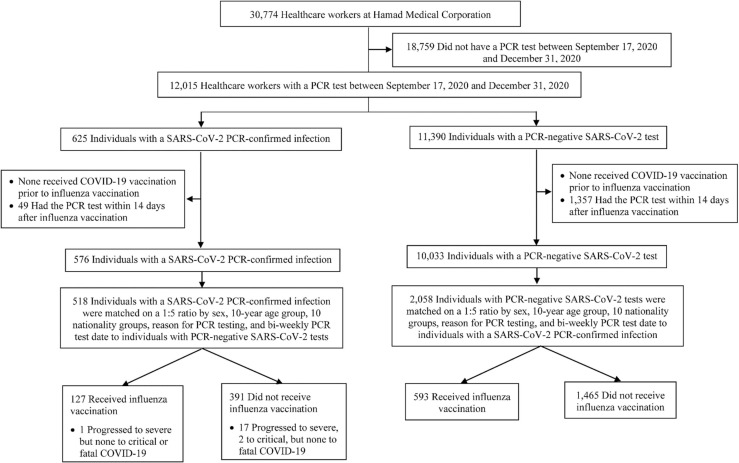
Fig. 1 shows the process of selecting the study population. Of 30,774 HCWs at Hamad Medical Corporation, only 12,015 individuals who had one or more SARS-CoV-2 PCR tests between September 17, 2020 and December 31, 2020 (study was designed to end before the start of COVID-19 mass vaccination campaigns) qualified for further investigation in this study. Of these, 576 HCWs with a PCR-positive test and 10,033 with exclusively PCR-negative tests met the study inclusion criteria. Exact-matching in a 1:5 ratio yielded 518 cases matched to 2058 controls that were included in the analysis.
Fig. 1.
Flowchart describing the population selection process for investigating effectiveness of influenza vaccination against SARS-CoV-2 infection.
Table 1 describes characteristics of cases and controls. The median age was 36 years (interquartile range (IQR), 32–44) for cases and 35 years (IQR, 32–42) for controls in the matched samples. Slightly less than half (∼ 46%) of cases and controls were males. Study participants were HCWs residing in Qatar, but of diverse nationalities. There were 31 different nationalities among matched cases and 47 different nationalities among matched controls (Table 1).
Table 1.
Characteristics of cases (healthcare workers with PCR-positive tests) and controls (healthcare workers with PCR-negative tests).
| Characteristics | All cases and controls (unmatched) |
Matched cases and controlsa |
||||
|---|---|---|---|---|---|---|
| Cases (PCR-confirmed infection) |
Controls (PCR-negative) |
SMDb | Cases (PCR-confirmed infection) |
Controls (PCR-negative) |
SMDb | |
| No | 576 | 10,033 | 518 | 2058 | ||
| Median age (IQR) — years | 37 (32–45) | 36 (32–44) | 0.04c | 36 (32–44) | 35 (32–42) | 0.11c |
| Age group — no. (%) | ||||||
| < 30 years | 80 (13.9) | 1370 (13.7) | 0.13 | 65 (12.6) | 233 (11.3) | 0.18 |
| 30–39 years | 261 (45.3) | 4973 (49.6) | 255 (49.2) | 1152 (56.0) | ||
| 40–49 years | 145 (25.2) | 2354 (23.5) | 128 (24.7) | 496 (24.1) | ||
| 50–59 years | 81 (14.1) | 1120 (11.2) | 66 (12.7) | 171 (8.3) | ||
| 60–69 years | 9 (1.6) | 201 (2.0) | 4 (0.8) | 6 (0.3) | ||
| 70 + years | 0 (0.0) | 15 (0.2) | -- | -- | ||
| Sex | ||||||
| Male | 270 (46.9) | 4949 (49.3) | 0.05 | 243 (46.9) | 951 (46.2) | 0.01 |
| Female | 306 (53.1) | 5084 (50.7) | 275 (53.1) | 1107 (53.8) | ||
| Nationalityd | ||||||
| Bangladeshi | 1 (0.2) | 77 (0.8) | 0.15 | -- | -- | 0.15 |
| Egyptian | 30 (5.2) | 510 (5.1) | 21 (4.1) | 62 (3.0) | ||
| Filipino | 78 (13.5) | 1637 (16.3) | 72 (13.9) | 271 (13.2) | ||
| Indian | 196 (34.0) | 3095 (30.9) | 185 (35.7) | 814 (39.6) | ||
| Nepalese | 6 (1.0) | 45 (0.5) | 1 (0.2) | 4 (0.2) | ||
| Pakistani | 11 (1.9) | 223 (2.2) | 6 (1.2) | 11 (0.5) | ||
| Qatari | 78 (13.5) | 1412 (14.1) | 74 (14.3) | 262 (12.7) | ||
| Sri Lankan | 1 (0.2) | 22 (0.2) | -- | -- | ||
| Sudanese | 24 (4.2) | 347 (3.5) | 18 (3.5) | 43 (2.1) | ||
| Other nationalitiese | 151 (26.2) | 2665 (26.6) | 141 (27.2) | 591 (28.7) | ||
| Reason for PCR testing | ||||||
| Clinical suspicion | 320 (55.6) | 3680 (36.7) | 0.57 | 299 (57.7) | 1264 (61.4) | 0.14 |
| Contact tracing | 105 (18.2) | 1197 (11.9) | 95 (18.3) | 346 (16.8) | ||
| Other survey testing | 13 (2.3) | 399 (4.0) | 10 (1.9) | 21 (1.0) | ||
| Port of entry | 52 (9.0) | 1359 (13.6) | 43 (8.3) | 172 (8.4) | ||
| Individual request | 18 (3.1) | 535 (5.3) | 11 (2.1) | 19 (0.9) | ||
| Survey testing | 37 (6.4) | 1081 (10.8) | 32 (6.2) | 128 (6.2) | ||
| Health care routine testing | 30 (5.2) | 1517 (15.1) | 27 (5.2) | 103 (5.0) | ||
| Pre-travel | 1 (0.2) | 265 (2.6) | 1 (0.2) | 5 (0.2) | ||
| Comorbidity count | ||||||
| 0 | 276 (47.9) | 5295 (52.8) | 0.11 | 254 (49.0) | 1099 (53.4) | 0.10 |
| 1 | 157 (27.3) | 2603 (25.9) | 142 (27.4) | 544 (26.4) | ||
| 2 | 82 (14.2) | 1175 (11.7) | 70 (13.5) | 251 (12.2) | ||
| ≥ 3 | 61 (10.6) | 960 (9.6) | 52 (10.0) | 164 (8.0) | ||
| COVID-19 severity | ||||||
| Asymptomatic, mild, or moderate COVID-19 | 551 (95.7) | -- | -- | 498 (96.1) | -- | -- |
| Severe COVID-19 | 23 (4.0) | -- | 18 (3.5) | -- | ||
| Critical COVID-19 | 2 (0.3) | -- | 2 (0.4) | -- | ||
| Fatal COVID-19 | 0 | -- | 0 | -- | ||
Abbreviations: IQR = interquartile range; PCR = polymerase chain reaction; SMD = standardized mean difference.
aCases and controls were exact-matched in a 1:5 ratio by sex, 10-year age group, 10 nationality groups, reason for PCR testing, and bi-weekly PCR test date.
bSMD is the difference in the mean of a covariate between groups divided by the pooled standard deviation.
cSMD reported here is for the mean difference between groups divided by the pooled standard deviation.
dNationalities were chosen to represent the most numerous groups in the population of Qatar.
eThere were 22 other nationalities in the total case population, 74 other nationalities in the total control population, 22 other nationalities among matched cases, and 38 other nationalities among matched controls. Matched cases included the following “other nationalities”: American, Australian, British, Canadian, Djiboutian, Eritrean, Ethiopian, German, Iranian, Iraqi, Irish, Jordanian, Lebanese, Nigerian, Omani, Palestinian, Slovenian, Somali, South African, Syrian, Tunisian, and Yemeni. Matched controls included the following “other nationalities”: Algerian, American, Australian, Austrian, Azerbaijani, Bahraini, British, Bulgarian, Canadian, Djiboutian, Eritrean, French, German, Ghanaian, Greek, Hungarian, Indonesian, Iranian, Iraqi, Irish, Jordanian, Lebanese, Libyan, Mauritius, Moroccan, New Zealander, Nigerian, Omani, Palestinian, Saudi, Serbian, Somali, South African, Syrian, Tanzanian, Tunisian, Turkish, and Yemeni.
Nearly two-thirds of study participants were tested because of COVID-like symptoms. Matched study groups were well balanced across matching factors. Among matched cases and controls, respectively, 49.0% versus 53.4% had 0 comorbidities, 27.4% versus 26.4% had 1 comorbidity, 13.5% versus 12.2% had 2 comorbidities, and 10.0% versus 8.0% had ≥ 3 comorbidities, with an SMD of 0.10 indicating adequate balance. The median duration between influenza vaccination and the PCR test was 43 days (IQR, 29–62).
The odds of having a record of influenza vaccination among PCR-positive cases compared to PCR-negative controls was 0.70 (95% CI: 0.52–0.95), indicating lower frequency of vaccination among cases compared to controls. Accordingly, the estimated effectiveness of influenza vaccination against SARS-CoV-2 infection> 14 days after receiving the vaccine was 29.7% (95% CI: 5.5–47.7%). All sensitivity analyses yielded consistent results, confirming main analysis results ( Table 2).
Table 2.
Effectiveness of influenza vaccination against SARS-CoV-2 infection> 14 days after vaccine administration and against any severe, critical, or fatal COVID-19.
| Analyses | Casesa (PCR-confirmed infection) |
Controlsa (PCR-negative tests) |
Adjusted odds ratio (95% CI) | Effectiveness in % (95% CI)b |
||
|---|---|---|---|---|---|---|
| Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | |||
| Main analysis-Effectiveness against SARS-CoV-2 infection | 127 | 391 | 593 | 1465 | 0.70 (0.52–0.95) |
29.7 (5.5–47.7) |
| Sensitivity analyses | ||||||
| Adjusting for prior infection in the conditional logistic regression | 127 | 391 | 593 | 1465 | 0.71 (0.53–0.96) |
28.6 (4.0–46.9) |
| Excluding prior infection | 126 | 385 | 576 | 1440 | 0.72 (0.54–0.96) |
28.1 (3.7–46.3) |
| Excluding travel-related PCR testing | 124 | 357 | 577 | 1336 | 0.72 (0.54–0.97) |
27.9 (3.2–46.3) |
| Including all PCR positive and PCR negative tests | 169 | 494 | 844 | 1917 | 0.66 (0.52–0.85) |
33.6 (15.0–48.2) |
| Severity analysis-Effectiveness against any severe, critical, or fatal COVID-19c | 1 | 19 | 18 | 62 | 0.11 (0.01–0.96) |
88.9 (4.1–98.7) |
Abbreviations: CI = confidence interval; COVID-19 = coronavirus disease 2019; PCR = polymerase chain reaction.
aCases and controls were exact-matched on a 1:5 ratio by sex, 10-year age group, 10 nationality groups, reason for PCR testing, and bi-weekly PCR test date.
Table 3 describes characteristics of vaccinated and unvaccinated matched PCR-positive cases. Of 127 matched PCR-positive cases who received influenza vaccination, only 1 progressed to severe disease, but none to critical or fatal COVID-19 (Fig. 1). Meanwhile, of the 391 matched PCR-positive cases who did not receive influenza vaccination, 17 progressed to severe, 2 to critical, but none to fatal COVID-19. The odds of having a record of influenza vaccination among severe, critical, or fatal COVID-19 cases compared to PCR-negative controls was 0.11 (95% CI: 0.01–0.96), indicating lower frequency of vaccination among cases compared to controls. Accordingly, the estimated effectiveness of influenza vaccination against any severe, critical, or fatal COVID-19 was 88.9% (95% CI: 4.1–98.7%; Table 2).
Table 3.
Characteristics of PCR-positive cases in the matched study sample stratified by influenza vaccination status.
| Characteristics | Vaccinated | Unvaccinated |
|---|---|---|
| No | 127 | 391 |
| Median age (IQR) — years | 36 (32–45) | 36 (32–44) |
| Age group — no. (%) | ||
| < 30 years | 9 (7.1) | 56 (14.3) |
| 30–39 years | 64 (50.4) | 191 (48.9) |
| 40–49 years | 37 (29.1) | 91 (23.3) |
| 50–59 years | 16 (12.6) | 50 (12.8) |
| 60–69 years | 1 (0.8) | 3 (0.8) |
| 70 + years | 0 (0.0) | 0 (0.0) |
| Sex | ||
| Male | 67 (52.8) | 176 (45.0) |
| Female | 60 (47.2) | 215 (55.0) |
| Nationalitya | ||
| Bangladeshi | 3 (2.4) | 18 (4.6) |
| Egyptian | 30 (23.6) | 42 (10.7) |
| Filipino | 64 (50.4) | 121 (31.0) |
| Indian | 0 (0.0) | 1 (0.3) |
| Nepalese | 1 (0.8) | 5 (1.3) |
| Pakistani | 3 (2.4) | 71 (18.2) |
| Qatari | 2 (1.6) | 16 (4.1) |
| Sri Lankan | 24 (18.9) | 117 (29.9) |
| Sudanese | 3 (2.4) | 18 (4.6) |
| Other nationalitiesb | 30 (23.6) | 42 (10.7) |
| Reason for PCR testing | ||
| Clinical suspicion | 89 (70.1) | 210 (53.7) |
| Contact tracing | 15 (11.8) | 80 (20.5) |
| Other survey testing | 3 (2.4) | 7 (1.8) |
| Port of entry | 4 (3.2) | 39 (10.0) |
| Individual request | 0 (0.0) | 11 (2.8) |
| Survey testing | 7 (5.5) | 25 (6.4) |
| Health care routine testing | 8 (6.3) | 19 (4.9) |
| Pre-travel | 1 (0.8) | 0 (0.0) |
Abbreviations: IQR = interquartile range; PCR = polymerase chain reaction.
aNationalities were chosen to represent the most numerous groups in the population of Qatar.
bThere were 10 other nationalities in the vaccinated group and 20 other nationalities in the unvaccinated group. Vaccinated cases included the following “other nationalities”: Australian, British, Eritrean, Iranian, Iraqi, Jordanian, Palestinian, Somali, Syrian, and Tunisian. Unvaccinated cases included the following “other nationalities”: American, British, Canadian, Djiboutian, Ethiopian, German, Iranian, Iraqi, Irish, Jordanian, Lebanese, Nigerian, Omani, Palestinian, Slovenian, Somali, South African, Syrian, Tunisian, and Yemeni.
Discussion
Recent influenza vaccination was associated with a 30% reduction in the risk of SARS-CoV-2 infection by the original virus, before introduction of variants of concern. Recent influenza vaccination was also associated with a 90% reduction in the risk of severe COVID-19, but the 95% confidence interval of the effectiveness estimate was wide. Frequency of severe COVID-19 was rare among those vaccinated, with only one case of severe COVID-19 documented among them.
These results for a HCW population in which the healthy user effect is perhaps minimized, support the conclusion that recent influenza vaccination has a genuine biological effect in protecting against SARS-CoV-2 infection and COVID-19 severity. These findings are consistent with findings of a systematic review conducted across 36 studies on nearly 56 million individuals [43]. The systematic review estimated the odds ratio comparing individuals with influenza vaccination to those without at 0.80 (95% CI: 0.73–0.87) against SARS-CoV-2 infection, 0.83 (95% CI: 0.72–0.96) against hospitalization in intensive care unit, 0.69 (95% CI: 0.57–0.84) against hospitalization for ventilator support, and 0.69 (95% CI: 0.52–0.93) against fatal COVID-19 [43].
The findings also corroborate findings of studies that found similar protective effects for influenza vaccination [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], though other studies did not [44], [45]. These findings, as informed by basic science laboratory investigations, may be explained by influenza vaccination triggering nonspecific immune activation, or trained or bystander immunity that is protective against SARS-CoV-2 infection [3], [4], [5], [7], [46], [47], [48], [49], [50], [51]. Further investigations are warranted to elucidate the exact mechanisms underlying the protective effect of influenza vaccination against COVID-19.
This study has limitations. Effectiveness of only recent influenza vaccination was investigated. The study used a retrospective design that analyzed available routinely collected data as opposed to a longitudinal study design where cohorts are recruited and followed over time. The analysis did not factor influenza vaccination from prior seasons. However, given that nonspecific immune activation may not last beyond few weeks, and in the context of the relatively rapid waning of COVID-19 vaccine immunity [18], [28], [29], [52], [53], [54], the observed effectiveness of influenza vaccination is likely short lived. Data were not available on vaccines for other infections (other than influenza and COVID-19 vaccines). However, there is no reason to believe that vaccination against any other infection could have differentially affected the matched cases and controls to affect our results.
Matching was done for sex, age, nationality, reason for PCR testing, and bi-weekly PCR test date, but it was not possible for other factors such as comorbidities, body mass index, use of immunosuppressive drugs and tonics such as zinc and vitamins, which were not available to the authors. However, matching factors used in this study successfully controlled bias in our earlier studies [18], [21], [23], [29], [39]. Nonetheless, one cannot exclude the possibility that in real-world data, bias could arise in unexpected ways, or from unknown sources, such as subtle differences or changes in test-seeking behavior. With the young and occupational nature of our population, these findings may not generalize to elderly persons or to the wider general population.
Notwithstanding these limitations, extensive sensitivity and additional analyses were conducted to investigate effects of potential bias in this study and in our earlier studies that used the same methodology. These included different adjustments and controls in the analysis and different study inclusion and exclusion criteria, to investigate whether effectiveness estimates could have been biased [18], [29]. These analyses showed consistent findings [18], [28], [29], [55], [56].
In conclusion, recent influenza vaccination is associated with a significant reduction in the risk of SARS-CoV-2 infection and COVID-19 severity. The findings support benefits for influenza vaccination that extend beyond protection against influenza infection and associated severe disease.
Funding
The authors are grateful for institutional salary support from the Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core, both at Weill Cornell Medicine-Qatar, as well as for institutional salary support provided by the Ministry of Public Health, Hamad Medical Corporation, and Sidra Medicine. Open access funding provided by the Qatar National Library. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the article. Statements made herein are solely the responsibility of the authors.
CRediT authorship contribution statement
ET, SA, MAA, and AAK co-conceived and co-designed the study, conducted literature review, co-developed the database, and co-wrote the first draft of the article. HC co-designed the study, co-developed the database, performed the statistical analyses, and co-wrote the first draft of the article. LJA co-designed the study, led the statistical analyses, and co-wrote the first draft of the article. All authors contributed to data collection and acquisition, database development, discussion and interpretation of the results, and to the writing of the manuscript. All authors have read and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We acknowledge the many dedicated individuals at Hamad Medical Corporation, the Ministry of Public Health, the Primary Health Care Corporation, Qatar Biobank, Sidra Medicine, and Weill Cornell Medicine-Qatar for their diligent efforts and contributions to make this study possible.
Ethics approval and consent to participate
Hamad Medical Corporation and Weill Cornell Medicine-Qatar Institutional Review Boards approved this retrospective study with a waiver of informed consent. The research was performed in accordance with relevant guidelines and regulations.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jiph.2022.12.016.
Appendix A. Supplementary material
Supplementary material.
.
References
- 1.Jackson M.L., Chung J.R., Jackson L.A., Phillips C.H., Benoit J., Monto A.S., et al. Influenza Vaccine Effectiveness in the United States during the 2015-2016 Season. N Engl J Med. 2017;377(6):534–543. doi: 10.1056/NEJMoa1700153. PubMed PMID: 28792867. Pubmed Central PMCID: PMC5727917. Epub 2017/08/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearson M.L., Bridges C.B., Harper S.A. Healthcare infection control practices advisory C, advisory committee on immunization P. Influenza vaccination of health-care personnel: recommendations of the healthcare infection control practices advisory committee (HICPAC) and the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-2):1–16. PubMed PMID: 16498385. Epub 2006/02/25. [PubMed] [Google Scholar]
- 3.Conlon A., Ashur C., Washer L., Eagle K.A., Hofmann, Bowman M.A. Impact of the influenza vaccine on COVID-19 infection rates and severity. Am J Infect Control. 2021;49(6):694–700. doi: 10.1016/j.ajic.2021.02.012. PubMed PMID: 33631305. Pubmed Central PMCID: PMC7899024. Epub 2021/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fink G., Orlova-Fink N., Schindler T., Grisi S., Ferrer A.P.S., Daubenberger C., et al. Inactivated trivalent influenza vaccination is associated with lower mortality among patients with COVID-19 in Brazil. BMJ Evid Based Med. 2020 doi: 10.1136/bmjebm-2020-111549. PubMed PMID: 33310766. Pubmed Central PMCID: PMC7735072. Epub 2020/12/15. [DOI] [PubMed] [Google Scholar]
- 5.Huang K., Lin S.W., Sheng W.H., Wang C.C. Influenza vaccination and the risk of COVID-19 infection and severe illness in older adults in the United States. Sci Rep. 2021;11(1):11025. doi: 10.1038/s41598-021-90068-y. PubMed PMID: 34040014. Pubmed Central PMCID: PMC8155195. Epub 2021/05/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taghioff S.M., Slavin B.R., Holton T., Singh D. Examining the potential benefits of the influenza vaccine against SARS-CoV-2: a retrospective cohort analysis of 74,754 patients. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0255541. PubMed PMID: 34343191. Pubmed Central PMCID: PMC8330918 Taghioff, and Dr. Singh have no relevant disclosures. The authors have not received any consulting fees, stock options, research funding, capital equipment, or educational grants from TriNetX. Epub 2021/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debisarun P.A., Gossling K.L., Bulut O., Kilic G., Zoodsma M., Liu Z., et al. Induction of trained immunity by influenza vaccination - impact on COVID-19. PLoS Pathog. 2021;17(10) doi: 10.1371/journal.ppat.1009928. PubMed PMID: 34695164. Pubmed Central PMCID: PMC8568262. Epub 2021/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ragni P., Marino M., Formisano D., Bisaccia E., Scaltriti S., Bedeschi E., et al. Association between exposure to influenza vaccination and COVID-19 diagnosis and outcomes. Vaccin (Basel) 2020;8(4) doi: 10.3390/vaccines8040675. PubMed PMID: 33198368. Pubmed Central PMCID: PMC7711765. Epub 2020/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marin-Hernandez D., Schwartz R.E., Nixon D.F. Epidemiological evidence for association between higher influenza vaccine uptake in the elderly and lower COVID-19 deaths in Italy. J Med Virol. 2021;93(1):64–65. doi: 10.1002/jmv.26120. PubMed PMID: 32497290. Pubmed Central PMCID: PMC7300995. Epub 2020/06/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massoudi N., Mohit B. A case-control study of the 2019 influenza vaccine and incidence of COVID-19 among healthcare workers. J Clin Immunol. 2021;41(2):324–334. doi: 10.1007/s10875-020-00925-0. PubMed PMID: 33244671. Pubmed Central PMCID: PMC7690338. Epub 2020/11/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawlowski C., Puranik A., Bandi H., Venkatakrishnan A.J., Agarwal V., Kennedy R., et al. Exploratory analysis of immunization records highlights decreased SARS-CoV-2 rates in individuals with recent non-COVID-19 vaccinations. Sci Rep. 2021;11(1):4741. doi: 10.1038/s41598-021-83641-y. PubMed PMID: 33637783. Pubmed Central PMCID: PMC7910541. Epub 2021/02/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Candelli M., Pignataro G., Torelli E., Gulli A., Nista E.C., Petrucci M., et al. Effect of influenza vaccine on COVID-19 mortality: a retrospective study. Intern Emerg Med. 2021;16(7):1849–1855. doi: 10.1007/s11739-021-02702-2. PubMed PMID: 33743150. Pubmed Central PMCID: PMC7980752. Epub 2021/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seif-Farshad M., Alizadeh M., Khayatzadeh S., Heidari F. The relationship of COVID-19 morbidity and mortality with the history of influenza vaccination. Med J Islam Repub Iran. 2022;36:122. doi: 10.47176/mjiri.36.122. PubMed PMID: 36447553. Pubmed Central PMCID: PMC9700421. Epub 2022/12/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson L.A., Jackson M.L., Nelson J.C., Neuzil K.M., Weiss N.S. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol. 2006;35(2):337–344. doi: 10.1093/ije/dyi274. PubMed PMID: 16368725. Epub 2005/12/22. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. COVID-19 clinical management: living guidance, https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021–1; 2021 [accessed May 31, 2021].
- 16.World Health Organization. International guidelines for certification and classification (coding) of COVID-19 as cause of death. Document Number: WHO/HQ/DDI/DNA/CAT, https://www.who.int/classifications/icd/Guidelines_Cause_of_Death_COVID-19–20200420-EN.pdf?ua=1; 2021 (Accessed 31 May 2021).
- 17.Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., Al Kanaani Z., Al Khal A., Al Kuwari E., et al. Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic. Sci Rep. 2021;11(1):6233. doi: 10.1038/s41598-021-85428-7. PubMed PMID: 33737535. Pubmed Central PMCID: PMC7973743. Epub 2021/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chemaitelly H., Tang P., Hasan M.R., AlMukdad S., Yassine H.M., Benslimane F.M., et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385(24) doi: 10.1056/NEJMoa2114114. PubMed PMID: 34614327. Pubmed Central PMCID: PMC8522799. Epub 2021/10/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chemaitelly H., Bertollini R., Abu-Raddad L.J. National study group for Covid epidemiology. Efficacy of natural immunity against SARS-CoV-2 reinfection with the Beta Variant. N Engl J Med. 2021;385(27):2585–2586. doi: 10.1056/NEJMc2110300. PubMed PMID: 34910864. Pubmed Central PMCID: PMC8693689. Epub 2021/12/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu-Raddad L.J., Chemaitelly H., Butt A.A. National study group for covid vaccination. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385(2):187–189. doi: 10.1056/NEJMc2104974. PubMed PMID: 33951357. Pubmed Central PMCID: PMC8117967. Epub 2021/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chemaitelly H., Yassine H.M., Benslimane F.M., Al Khatib H.A., Tang P., Hasan M.R., et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27(9):1614–1621. doi: 10.1038/s41591-021-01446-y. PubMed PMID: 34244681. Epub 2021/07/11. [DOI] [PubMed] [Google Scholar]
- 22.Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., AlMukdad S., Yassine H.M., Al-Khatib H.A., et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N Engl J Med. 2022;386(19):1804–1816. doi: 10.1056/NEJMoa2200797. PubMed PMID: 35263534. Pubmed Central PMCID: PMC8929389. Epub 2022/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abu-Raddad L.J., Chemaitelly H., Bertollini R. National study group for Covid vaccination. Effectiveness of mRNA-1273 and BNT162b2 Vaccines in Qatar. N Engl J Med. 2022;386(8):799–800. doi: 10.1056/NEJMc2117933. PubMed PMID: 35045222. Pubmed Central PMCID: PMC8796790. Epub 2022/01/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altarawneh H.N., Chemaitelly H., Ayoub H.H., Tang P., Hasan M.R., Yassine H.M., et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387(1):21–34. doi: 10.1056/NEJMoa2203965. PubMed PMID: 35704396. Pubmed Central PMCID: PMC9258753. Epub 2022/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson M.L., Nelson J.C. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31(17):2165–2168. doi: 10.1016/j.vaccine.2013.02.053. PubMed PMID: 23499601. Epub 2013/03/19. [DOI] [PubMed] [Google Scholar]
- 26.Verani J.R., Baqui A.H., Broome C.V., Cherian T., Cohen C., Farrar J.L., et al. Case-control vaccine effectiveness studies: Preparation, design, and enrollment of cases and controls. Vaccine. 2017;35(25):3295–3302. doi: 10.1016/j.vaccine.2017.04.037. PubMed PMID: 28442231. Pubmed Central PMCID: PMC7007298. Epub 2017/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang P., Hasan M.R., Chemaitelly H., Yassine H.M., Benslimane F.M., Al Khatib H.A., et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 delta variant in Qatar. Nat Med. 2021;27(12):2136–2143. doi: 10.1038/s41591-021-01583-4. PubMed PMID: 34728831. Epub 2021/11/04. [DOI] [PubMed] [Google Scholar]
- 28.Chemaitelly H., Ayoub H.H., AlMukdad S., Coyle P., Tang P., Yassine H.M., et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun. 2022;13(1):3082. doi: 10.1038/s41467-022-30895-3. PubMed PMID: 35654888. Epub 2022/06/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-Raddad L.J., Chemaitelly H., Bertollini R. National study group for covid vaccination. Waning mRNA-1273 vaccine effectiveness against SARS-CoV-2 infection in Qatar. N Engl J Med. 2022;386(11):1091–1093. doi: 10.1056/NEJMc2119432. PubMed PMID: 35081294. Pubmed Central PMCID: PMC8809505. Epub 2022/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., Coyle P., Malek J.A., Ahmed A.A., et al. Introduction and expansion of the SARS-CoV-2 B.1.1.7 variant and reinfections in Qatar: a nationally representative cohort study. PLoS Med. 2021;18(12) doi: 10.1371/journal.pmed.1003879. PubMed PMID: 34914711. Pubmed Central PMCID: PMC8726501 following competing interests: A.A.B. has received institutional grant funding from Gilead Sciences unrelated to the work presented in this paper, otherwise, the authors declare no competing interests. Epub 2021/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Project of Surveillance for Variants of Concern and Viral Genome Sequencing. Qatar viral genome sequencing data. Data on randomly collected samples, https://www.gisaid.org/phylodynamics/global/nextstrain/; 2021 (Accessed 31 May 2021).
- 32.Benslimane F.M., Al Khatib H.A., Al-Jamal O., Albatesh D., Boughattas S., Ahmed A.A., et al. One year of SARS-CoV-2: genomic characterization of COVID-19 outbreak in Qatar. Front Cell Infect Microbiol. 2021;11 doi: 10.3389/fcimb.2021.768883. PubMed PMID: 34869069. Pubmed Central PMCID: PMC8637114. Epub 2021/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bsat R., Chemaitelly H., Coyle P., Tang P., Hasan M.R., Al Kanaani Z., et al. Characterizing the effective reproduction number during the COVID-19 pandemic: Insights from Qatar's experience. J Glob Health. 2022;12:05004. doi: 10.7189/jogh.12.05004. PubMed PMID: 35136602. Pubmed Central PMCID: PMC8819337 Sciences unrelated to the work presented in this paper. The authors have completed the ICMJE Declaration of Interest Form (available upon request from the corresponding author), and declare no further conflicts of interest. Epub 2022/02/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasan M.R., Kalikiri M.K.R., Mirza F., Sundararaju S., Sharma A., Xaba T., et al. Real-Time SARS-CoV-2 genotyping by high-throughput multiplex PCR reveals the epidemiology of the variants of concern in Qatar. Int J Infect Dis. 2021;112:52–54. doi: 10.1016/j.ijid.2021.09.006. PubMed PMID: 34525398. Epub 2021/09/16. [DOI] [PubMed] [Google Scholar]
- 35.Ayoub H.H., Chemaitelly H., Seedat S., Makhoul M., Al Kanaani Z., Al, et al. Mathematical modeling of the SARS-CoV-2 epidemic in Qatar and its impact on the national response to COVID-19. J Glob Health. 2021;16(11):05005. doi: 10.7189/jogh.11.05005. PubMed PMID: 33643638. Pubmed Central PMCID: PMC7897910 form (available upon request from the corresponding author), and declare no conflicts of interest. Epub 2021/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coyle P.V., Chemaitelly H., Ben Hadj Kacem M.A., Abdulla Al Molawi N.H., El Kahlout R.A., Gilliani I., et al. SARS-CoV-2 seroprevalence in the urban population of Qatar: an analysis of antibody testing on a sample of 112,941 individuals. iScience. 2021;24(6) doi: 10.1016/j.isci.2021.102646. PubMed PMID: 34056566. Pubmed Central PMCID: PMC8142077. Epub 2021/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Thani M.H., Farag E., Bertollini R., Al Romaihi H.E., Abdeen S., Abdelkarim A., et al. SARS-CoV-2 infection is at herd immunity in the majority segment of the population of Qatar. Open Forum Infect Dis. 2021;8(8):ofab221. doi: 10.1093/ofid/ofab221. PubMed PMID: 34458388. Pubmed Central PMCID: PMC8135898. Epub 2021/08/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeremijenko A., Chemaitelly H., Ayoub H.H., Alishaq M., Abou-Samra A.B., Al Ajmi J., et al. Herd Immunity against severe acute respiratory syndrome coronavirus 2 infection in 10 communities, Qatar. Emerg Infect Dis. 2021;27(5):1343–1352. doi: 10.3201/eid2705.204365. PubMed PMID: 33900174. Pubmed Central PMCID: PMC8084480. Epub 2021/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abu-Raddad L.J., Chemaitelly H., Yassine H.M., Benslimane F.M., Al Khatib H.A., Tang P., et al. Pfizer-BioNTech mRNA BNT162b2 Covid-19 vaccine protection against variants of concern after one versus two doses. J Travel Med. 2021;28(7) doi: 10.1093/jtm/taab083. PubMed PMID: 34050372. Pubmed Central PMCID: PMC8194836. Epub 2021/05/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin P.C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. communications in statistics - simulation and computation. 2009;38(6):1228–1234. 2009/05/14. [Google Scholar]
- 41.Jacoby P., Kelly H. Is it necessary to adjust for calendar time in a test negative design?: Responding to: Jackson ML, Nelson JC. The test negative design for estimating influenza vaccine effectiveness. Vaccine 2013;31(April (17)):2165-8. Vaccine. 2014;32(25):2942. doi: 10.1016/j.vaccine.2013.08.048. PubMed PMID: 24016812. Epub 2013/09/11. [DOI] [PubMed] [Google Scholar]
- 42.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. Jama. 2013 Nov 27;310(20):2191–4. PubMed PMID: 24141714. Epub 2013/10/22. eng. [DOI] [PubMed]
- 43.Jiang B., Huang Q., Jia M., Xue X., Wang Q., Yang W., et al. Association between influenza vaccination and SARS-CoV-2 infection and its outcomes: systematic review and meta-analysis. Chin Med J. 2022 doi: 10.1097/CM9.0000000000002427. PubMed PMID: 36378238. Epub 2022/11/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belingheri M., Paladino M.E., Latocca R., De Vito G., Riva M.A. Association between seasonal flu vaccination and COVID-19 among healthcare workers. Occup Med (Lond) 2020;70(9):665–671. doi: 10.1093/occmed/kqaa197. PubMed PMID: 33300998. Pubmed Central PMCID: PMC7798744. Epub 2020/12/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kissling E., Hooiveld M., Brytting M., Vilcu A.M., de Lange M., Martinez-Baz I., et al. Absence of association between 2019-20 influenza vaccination and COVID-19: results of the European I-MOVE-COVID-19 primary care project, March-August 2020. Influenza Other Respir Virus. 2021;15(4):429–438. doi: 10.1111/irv.12839. PubMed PMID: 33481344. Pubmed Central PMCID: PMC8013620. Epub 2021/01/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erismis B., Karabela S.N., Eksi F., Karandere F., Dogan B., Okay F., et al. Annual influenza vaccination effect on the susceptibility to COVID-19 infection. Cent Eur J Public Health. 2021;29(1):14–17. doi: 10.21101/cejph.a6573. PubMed PMID: 33831281. Epub 2021/04/09. [DOI] [PubMed] [Google Scholar]
- 47.Horns F., Dekker C.L., Quake S.R., Memory B. Cell activation, broad anti-influenza antibodies, and bystander activation revealed by single-cell transcriptomics. Cell Rep. 2020;30(3):905–913. doi: 10.1016/j.celrep.2019.12.063. e6. PubMed PMID: 31968262. Pubmed Central PMCID: PMC7891556. Epub 2020/01/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salem M.L., El-Hennawy D. The possible beneficial adjuvant effect of influenza vaccine to minimize the severity of COVID-19. Med Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109752. PubMed PMID: 32361099. Pubmed Central PMCID: PMC7194943. Epub 2020/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee C.H., Pinho M.P., Buckley P.R., Woodhouse I.B., Ogg G., Simmons A., et al. Potential CD8+ T cell cross-reactivity against SARS-CoV-2 conferred by other coronavirus strains. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.579480. PubMed PMID: 33250893. Pubmed Central PMCID: PMC7676914. Epub 2020/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benn C.S., Netea M.G., Selin L.K., Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34(9):431–439. doi: 10.1016/j.it.2013.04.004. PubMed PMID: 23680130. Epub 2013/05/18. [DOI] [PubMed] [Google Scholar]
- 51.Netea M.G., Dominguez-Andres J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–388. doi: 10.1038/s41577-020-0285-6. PubMed PMID: 32132681. Pubmed Central PMCID: PMC7186935. Epub 2020/03/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feikin D.R., Higdon M.M., Abu-Raddad L.J., Andrews N., Araos R., Goldberg Y., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–944. doi: 10.1016/S0140-6736(22)00152-0. PubMed PMID: 35202601. Pubmed Central PMCID: PMC8863502. Epub 2022/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chemaitelly H., Abu-Raddad L.J. Waning effectiveness of COVID-19 vaccines. Lancet. 2022;399(10327):771–773. doi: 10.1016/S0140-6736(22)00277-X. PubMed PMID: 35219385. Pubmed Central PMCID: PMC8871492. Epub 2022/02/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andrews N., Tessier E., Stowe J., Gower C., Kirsebom F., Simmons R., et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med. 2022;386(4):340–350. doi: 10.1056/NEJMoa2115481. PubMed PMID: 35021002. Pubmed Central PMCID: PMC8781262. Epub 2022/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ayoub H.H., Tomy M., Chemaitelly H., Altarawneh H.N., Coyle P., Tang P., et al. Estimating protection afforded by prior infection in preventing reinfection: Applying the test-negative study design. medRxiv. 2022:2022.01.02.22268622. [DOI] [PMC free article] [PubMed]
- 56.Altarawneh H.N., Chemaitelly H., Hasan M.R., Ayoub H.H., Qassim S., AlMukdad S., et al. Protection against the omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 2022;386(13):1288–1290. doi: 10.1056/NEJMc2200133. PubMed PMID: 35139269. Pubmed Central PMCID: PMC8849180. Epub 2022/02/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.