Abstract
Acute vasodilator testing (AVT) identifies acute responders for initiation of calcium channel blockers in pulmonary arterial hypertension (PAH) and operability in congenital heart disease (CHD). We sought to determine the feasibility of intravenous sildenafil (ivS) as an alternative to inhaled nitric oxide (iNO) in AVT. All patients with PAH undergoing cardiac catheterization for AVT (November 2015 to December 2020) were prospectively enrolled. Hemodynamic data were obtained at baseline, with iNO 20 ppm and ivS (0.25 mg/kg for children and 10 mg for adults). We studied 44 patients with a mean age of 20.5 ± 14.4 years (27 [61%] females and 20 [45%] children). There were 17 (38.6%) patients in the CHD group for operability assessment and 27 patients in non‐CHD group (idiopathic pulmonary arterial hypertension—16 [36.3%], residual PAH after shunt closure—7 [15.9%], and 2 cases [4.5%] each of familial PAH and portopulmonary hypertension). There was an excellent intraclass correlation for mean pulmonary artery pressures (0.903, 95% confidence interval, CI: 0.809–0.949, p < 0.001), mean aortic pressures (0.745, 95% CI: 0.552–0.858, p < 0.001), pulmonary vascular resistance index (0.920, 95% CI: 0.858–0.956, p < 0.001), systemic vascular resistance (SVR) index (0.828, 95% CI: 0.706–0.902, p < 0.001), and the ratio of pulmonary and SVR indices (0.857, 95% CI: 0.752–0.919, p < 0.001) between the two agents. There were two responders, both in non‐CHD group, and were identified by iNO and ivS. The hemodynamic effects of ivS show excellent correlation with iNO and could be a potential alternative agent for identifying acute responders during AVT.
Keywords: acute vasodilator test, cardiac catheterization, nitric oxide, pulmonary hypertension, sildenafil
INTRODUCTION
Pulmonary hypertension is a condition affecting pulmonary vasculature denovo or secondary to various identifiable causes. 1 Acute vasodilatory test (AVT) is used to identify acute responders in specific categories of pulmonary hypertension during cardiac catheterization. Acute responders are known to have better outcomes in comparison with nonresponders. 2 , 3 , 4 AVT is classically indicated in patients with idiopathic pulmonary arterial hypertension (IPAH), familial PAH, drug‐induced PAH, and assessment of operability in congenital heart disease (CHD) with elevated pulmonary vascular resistance (PVR).
The standard of care agent for AVT is inhaled nitric oxide (iNO). 5 , 6 , 7 , 8 , 9 , 10 The use of iNO requires a complicated delivery system, monitoring equipment, and personnel, is expensive and its availability is limited to selected centers. 5 , 6 It is particularly difficult to use iNO in limited resource environments because of cost considerations and lack of expertise in its use. Sildenafil, a phosphodiesterase 5 inhibitor has proven vasodilator properties and a good safety profile along with ease of availability and use in patients with PAH. 11 , 12 , 13 , 14 We hypothesized that intravenous sildenafil (ivS) could be used as an alternative to iNO for AVT in patients with PAH.
METHODS
This was a prospective cross‐sectional analytical study from November 2015 to December 2020 at a tertiary referral hospital in South India. The primary objective of this study was to compare the effect of ivS and iNO on systemic and pulmonary hemodynamics in patients with PAH during AVT. The secondary objective was to compare the ability of ivS with iNO in identifying acute responders during AVT in patients with PAH.
The inclusion criterion was all treatment naïve patients more than 3 months of age with Group 1 PAH undergoing cardiac catheterization for AVT. For the operability assessment of CHD, those with disproportionate PAH as per clinical and echocardiographic evaluation without systemic desaturation were included. We excluded all patients having CHD with univentricular physiology. PAH was defined as mean pulmonary artery pressure (mPAP) of ≥25 mmHg, PVR of >3 Wood units (Wu) in adults (indexed PVR [PVRI] of >3 Wu·m2 in pediatric age group), and pulmonary capillary wedge pressure <15 mmHg. 15 The pediatric age group was defined as age <18 years at the time of cardiac catheterization.
Calculation of sample size
The sample size was calculated from a pilot study of 15 patients. The study reported an intraclass correlation (ICC) of 0.7 for mPAP between the two agents. The minimum calculated sample size assuming a 95% confidence interval (CI) and 90% power was 17 patients. We recruited as many patients as we could during the study period considering the possible subgroup comparisons.
Cardiac catheterization protocol
The study was conducted in the cardiac catheterization laboratory (Artis Zee; Siemens) under local anesthesia and intravenous sedation with midazolam if required. The patients were spontaneously breathing using a well‐fitting nonrebreathing face mask secured with straps and connected to the anesthesia machine using a closed circuit breathing system with a pressure support mode of ventilation. The GE Avance CS2 anesthesia workstation (Wipro GE) was used to monitor the minute ventilation and end‐tidal CO2 levels. The standard dose of 20 ppm of iNO was delivered into the inspiratory limb of the circuit, from the flowmeter of the NO cylinder which contained 982 ppm of NO in pure nitrogen. iNO was routinely administered with fractional inspired oxygen (Fio2) of 25% to prevent hypoxemia. The desired NO flow in liters per minute was calculated by the following formula:
Initially, cardiac catheterization was done in room air to obtain baseline pressures and oximetry data. The same parameters were then recorded after 10 min of iNO administered at 20 ppm as explained above. After measuring hemodynamics under iNO, it was stopped, and 15 min of washout was given. The patient was then administered ivS at a dose of 10 mg for adults and 0.25 mg/kg up to a maximum adult dose of 10 mg for children as a slow infusion over 10 min. To maintain a consistent hemodynamic status, the second set of baseline parameters were obtained before administration of ivS if the mean aortic pressure (mAP) changed more than 5% from baseline after iNO washout. The dosage for children was determined from a series of studies in children receiving ivS ranging from 0.05 to 0.45 mg/kg for various PAH indications. 13 , 14 , 16 , 17 , 18 , 19 We empirically chose a safe in‐between dose of 0.25 mg/kg. The hemodynamics were assessed 20 min after ivS infusion to allow achievement of peak plasma level of sildenafil. 18
The pressures were measured using electromechanical transducers (Edwards Lifesciences), calibrated for each study, and oximetry was instantly analyzed with ABL800 FLEX analyzer (Radiometer Medical ApS). The hemodynamic variables measured were pulmonary artery pressures, aortic pressures, right atrial (RA) pressures, left atrial pressures (pulmonary capillary wedge pressure in intact atrial septum), and oximetry samples were collected from superior venacava, inferior venacava, pulmonary artery, aorta, and pulmonary vein (in cases with intact atrial septum pulmonary vein saturation was assumed to be same as aortic saturation or 98% in absence and presence of right to left shunt, respectively). The cardiac output was calculated using Fick's principle and oxygen consumption was obtained from the LaFarge table. The other derived hemodynamic variables were calculated using standard equations. The systemic blood flow/cardiac output (Qs), pulmonary blood flow (Qp), PVR, systemic vascular resistance (SVR), and pulmonary artery capacitance (PAC) were indexed to body surface area. Any use of Fio2 > 21%, mandated the inclusion of dissolved oxygen in shunt calculation by Fick's principle. In addition, we collected basic demographics, anthropometry, and clinical details. We used the Sitbon criteria (a reduction in mPAP of ≥10 mmHg reaching an absolute mPAP value of ≤40 mmHg with an increased or unchanged cardiac output from baseline) to define adult and pediatric acute responders. 20 , 21 An acute responder for operability in CHD was defined by a reduction in PVRI to <4 Wu·m2 and PVRI:indexed SVR (SVRI) ratio to <0.3 from the baseline. 22
Statistical analyses
Statistical analyses were done using SPSS Version 20.0 for Windows (IBM Corporation). The categorical variables were summarized using percentages and continuous variables using mean with standard deviation. We used the paired t test to compare the various hemodynamic parameters between the two agents. Wilcoxon signed rank test was used to compare the percentage change values from the baseline by two agents. We selected a two‐way mixed effect model (absolute agreement, single measure) ICC to find the agreement between iNO and ivS. A p value ≤ 0.05 was considered statistically significant.
RESULTS
Patient characteristics
We enrolled a total of 44 patients, 27 (61%) females and 20 (45%) children for the study. The mean age was 20.5 ± 14.4 years. Three patients (7%) had Down Syndrome. The detailed diagnosis is given in Table 1. All patients had normal acid–base balance and partial pressure of carbon dioxide levels during the study.
Table 1.
Baseline demographics
| N = 44 | |
|---|---|
| Age, years | 20.45 ± 14.4 |
| Females | 27 (61) |
| Children | 20 (45) |
| Weight, kg | 39.2 ± 20 |
| Height, cm | 141.4 ± 29.4 |
| BSA, m2 | 1.21 ± 0.4 |
| pH | 7.41 ± 0.05 |
| pCO2, mmHg | 41.61 ± 5.2 |
| Diagnosis | |
| CHD | 17 (38.6) |
| ASD | 11 (25) |
| AVSD | 4 (9) |
| VSD | 2 (4.6) |
| IPAH | 16 (36.3) |
| Residual PAH | 7 (15.9) |
| Familial PAH | 2 (4.5) |
| Portopulmonary hypertension | 2 (4.5) |
Note: Values are N (%), mean ± SD.
Abbreviations: ASD, atrial septal defect; AVSD, atrioventricular septal defect; BSA, body surface area; CHD, congenital heart disease; IPAH, idiopathic pulmonary arterial hypertension; PAH, pulmonary arterial hypertension; pCO2, partial pressure of carbon dioxide, VSD, ventricular septal defect.
Hemodynamics—at baseline, with iNO and ivS
At baseline, the mPAP was 57.9 ± 15.8 mmHg and the mAP was 87.8 ± 15.5 mmHg. The mean PVRI was 17.7 ± 10.6 Wu·m2, the mean SVRI 31.4 ± 11.4 Wu·m2, and mean PVRI:SVRI ratio 0.6 ± 0.3. The absolute values for all the variables at baseline and on both agents are presented in Table 2. We calculated the percentage change for each hemodynamic variable from the baseline for both agents. The value obtained at baseline was subtracted from the value obtained with the two agents and the percentage change from baseline was computed. A decrease in the hemodynamic variable from the baseline was denoted as a negative percentage change and an increase from the baseline as a positive percentage change (Table 3). When compared to iNO, ivS resulted in a greater fall in PA pressures (systolic, diastolic, and mean), aortic pressures (systolic and mean), and PVRI which were statistically significant. All the other hemodynamic parameters were comparable between the two agents (Table 3). The overall difference in percentage change of variables between the two agents ranged from 0.2% to 7.5%. All the measured and calculated hemodynamic variables showed statistically significant‐excellent ICC between iNO and ivS (Figure 1). All the patients tolerated ivS well with no adverse hemodynamic effects.
Table 2.
Values of hemodynamic variables at baseline, on iNO and ivS
| Hemodynamic variables (N = 44) | Baseline | iNO | ivS | p Value |
|---|---|---|---|---|
| sPAP, mmHg | 87.2 ± 20.6 | 78.3 ± 21.8 | 74.1 ± 22.1 | 0.007 |
| dPAP, mmHg | 40.0 ± 14.4 | 35.1 ± 14.2 | 33.1 ± 13.3 | 0.111 |
| mPAP, mmHg | 57.9 ± 15.8 | 51.7 ± 16.1 | 49.0 ± 15.2 | 0.008 |
| sAP, mmHg | 125.5 ± 22.5 | 122.9 ± 24.6 | 116.3 ± 20.9 | <0.001 |
| dAP, mmHg | 67.9 ± 13.3 | 68.6 ± 13.4 | 65.6 ± 11.6 | 0.027 |
| mAP, mmHg | 87.8 ± 15.5 | 86.8 ± 16.3 | 82.6 ± 13.7 | 0.068 |
| Qpi, L/min/m2 | 3.8 ± 3.1 | 3.8 ± 2.9 | 3.8 ± 2.5 | 0.726 |
| Qsi, L/min/m2 | 2.7 ± 1.1 | 2.8 ± 0.9 | 2.7 ± 0.9 | 0.401 |
| Qp:Qs | 1.4 ± 0.7 | 1.4 ± 0.8 | 1.5 ± 0.9 | 0.480 |
| PVRI, Wu·m2 | 17.7 ± 10.6 | 14.8 ± 9.5 | 14.0 ± 8.9 | 0.139 |
| SVRI, Wu·m2 | 31.4 ± 11.4 | 29.1 ± 9.7 | 28.5 ± 10.5 | 0.517 |
| PVRI:SVRI | 0.6 ± 0.3 | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.944 |
| PACI, mmHg/ml/min/m2 | 0.9 ± 0.7 | 1.0 ± 0.8 | 1.1 ± 0.7 | 0.230 |
| mRA, mmHg | 6.4 ± 3.0 | 5.9 ± 2.6 | 5.7 ± 2.4 | 0.130 |
| mLA, mmHg | 8.3 ± 3.0 | 8.0 ± 3.2 | 8.0 ± 3.3 | 0.884 |
| sPAP:sAP | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.914 |
| mPAP:mAP | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.645 |
Note: Values as mean ± SD.
Abbreviations: dAP, diastolc aortic pressure; dPAP, diastolic pulmonary artery pressure; iNO, inhaled nitric oxide; ivS, intravenous sildenafil; mAP, mean aortic pressure; mLA, mean left atrial pressure; mPAP, mean pulmonary artery pressure; mRA, mean right atrial pressure; PACI, indexed pulmonary artery capacitance; PVRI, indexed pulmonary vascular resistance; Qp, pulmonary blood flow; Qpi, indexed pulmonary blood flow; Qs, systemic blood flow; Qsi, indexed systemic blood flow; sAP, systolic aortic pressure; sPAP, systolic pulmonary artery pressure; SVRI, indexed systemic vascular resistance; Wu, wood units.
Table 3.
Percentage change of hemodynamic variables from baseline for iNO and ivS
| Hemodynamic variables (N = 44) | iNO | ivS | Difference of percentage change | p Value |
|---|---|---|---|---|
| sPAP | −9.9 ± 14.8 | −15.3 ± 13.6 | 5.5 | 0.002 |
| dPAP | −10.4 ± 19.4 | −16.1 ± 19.4 | 5.7 | 0.010 |
| mPAP | −10.2 ± 14.9 | −15.4 ± 13.2 | 5.2 | <0.001 |
| sAP | −2.1 ± 7.3 | −6.8 ± 10.0 | 4.7 | <0.001 |
| dAP | 1.4 ± 10.5 | −1.9 ± 16.3 | 3.3 | 0.139 |
| mAP | −1.0 ± 9.2 | −5.0 ± 13.2 | 3.9 | 0.018 |
| Qpi | 3.0 ± 13.7 | 2.6 ± 13.7 | 0.4 | 0.867 |
| Qsi | 4.9 ± 19.7 | 3.1 ± 20.8 | 1.8 | 0.796 |
| Qp:Qs | 0.7 ± 14.5 | 3.1 ± 20.9 | −2.4 | 0.706 |
| PVRI | −13.5 ± 20.7 | −19.3 ± 19.0 | 5.9 | 0.010 |
| SVRI | −3.6 ± 19.9 | −4.6 ± 27.5 | 1.0 | 0.339 |
| PVRI:SVRI | −5.7 ± 32.0 | −5.5 ± 35.3 | −0.2 | 0.733 |
| PACI | 21.6 ± 48.1 | 29.1 ± 41.5 | −7.5 | 0.213 |
| mRA | −4.2 ± 20.7 | −7.0 ± 20.7 | −2.8 | 0.150 |
| mLA | −3.3 ± 14.4 | −2.9 ± 17.8 | −0.4 | 0.872 |
| sPAP:sAP | −7.5 ± 16.2 | −8.4 ± 16.1 | 0.9 | 0.741 |
| mPAP:mAP | −8.9 ± 15.9 | −9.1 ± 18.5 | 0.2 | 0.762 |
Note: The numerical values denote percentage change for each hemodynamic variable, a negative value indicates a fall, and a positive value indicates an increase from the baseline. Values as mean ± SD.
Abbreviations: dAP, diastolc aortic pressure; dPAP, diastolic pulmonary artery pressure; iNO, inhaled nitric oxide; ivS, intravenous sildenafil; mAP, mean aortic pressure; mLA, mean left atrial pressure; mPAP, mean pulmonary artery pressure; mRA, mean right atrial pressure; PACI, indexed pulmonary artery capacitance; PVRI, indexed pulmonary vascular resistance; Qp, pulmonary blood flow; Qpi, indexed pulmonary blood flow; Qs, systemic blood flow; Qsi, indexed systemic blood flow; sAP, systolic aortic pressure; sPAP, systolic pulmonary artery pressure; SVRI, indexed systemic vascular resistance.
Figure 1.
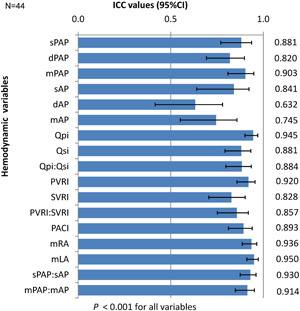
Intraclass correlation (ICC) between iNO and ivS for the whole cohort. X‐axis shows ICC values and 95% confidence intervals (CIs) marked by error bars. Y‐axis shows hemodynamic variables on the left‐hand side and ICC numerical values on the right‐hand side. dAP, diastolic aortic pressure; dPAP, diastolic pulmonary artery pressure; iNO, inhaled nitric oxide; ivS, intravenous sildenafil; mAP, mean aortic pressure; mLA, mean left atrial pressure; mPAP, mean pulmonary artery pressure; mRA, mean right atrial pressure; PACI, indexed pulmonary artery capacitance; PVRI, indexed pulmonary vascular resistance; Qpi, indexed pulmonary blood flow; Qsi, indexed systemic blood flow; sAP, systolic aortic pressure; sPAP, systolic pulmonary artery pressure; SVRI, indexed systemic vascular resistance.
Hemodynamics among responders
We categorized the cohort into two groups for responder identification—non‐CHD group (N = 27) for identification of acute responders and the CHD group (N = 17) for assessment of operability. There were two patients with IPAH (one child [1/14, 7.1%] and one adult [1/13, 7.6%]) meeting the criteria for an acute responder with iNO in the non‐CHD group, both were identified by ivS as well (Table 4). Among the CHD group, none met the criteria for operability, hence no acute responder was identified. Both groups had significant ICC between the two agents for all variables (Figure 2). Overall in the non‐CHD group, ivS caused a greater fall in pulmonary artery pressures, aortic pressures, PVRI, and a greater increase in PACI when compared to iNO though significant changes were observed only in mPAP and sAP (Figure 3). The changes in all other variables were comparable. The overall difference in percentage change of variables between the two agents ranged from 0% to 5.2%. In the CHD group, ivS produced a significant fall in PA pressures (systolic, diastolic, and mean) and aortic pressures (systolic and diastolic) when compared to iNO. There was comparable a fall in systemic cardiac output by both agents. The changes in other parameters were insignificant (Figure 4). The overall difference in percentage change of variables between the two agents ranged from 0% to 11.1%.
Table 4.
Data for acute responders
| N = 2 | Patient 1 | Patient 2 | ||||
|---|---|---|---|---|---|---|
| Age (years) | 11 | 26 | ||||
| Sex | Male | Female | ||||
| Diagnosis | IPAH | IPAH | ||||
| Hemodynamic variables | Baseline | iNO | ivS | Baseline | iNO | ivS |
| mPAP (mmHg) | 60 | 26 | 28 | 48 | 28 | 30 |
| Qsi (L/min/m2) | 3.2 | 3.5 | 4.1 | 1.9 | 1.9 | 2.1 |
| PVRI (Wu·m2) | 15 | 4.2 | 4.2 | 18.9 | 8.3 | 10.1 |
| PVRI:SVRI | 0.5 | 0.2 | 0.2 | 0.5 | 0.2 | 0.3 |
Abbreviations: iNO, inhaled nitric oxide; IPAH, idiopathic pulmonary arterial hypertension; ivS, intravenous sildenafil; mPAP, mean pulmonary artery pressure; PVRI, indexed pulmonary vascular resistance; Qsi, indexed systemic blood flow; SVRI, indexed systemic vascular resistance; Wu, Wood units.
Figure 2.
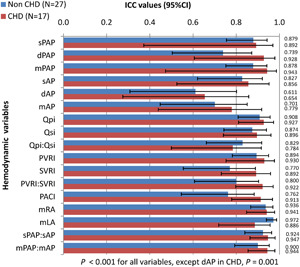
Intraclass correlation (ICC) between iNO and ivS for non‐CHD and CHD groups. X‐axis shows ICC values and 95% confidence intervals (CIs) marked by error bars. Y‐axis shows hemodynamic variables on the left‐hand side and ICC numerical values on the right‐hand side. CHD, congenital heart disease; dAP, diastolic aortic pressure; dPAP, diastolic pulmonary artery pressure; iNO, inhaled nitric oxide; ivS, intravenous sildenafil; mAP, mean aortic pressure; mLA, mean left atrial pressure; mPAP, mean pulmonary artery pressure; mRA, mean right atrial pressure; PACI, indexed pulmonary artery capacitance; PVRI, indexed pulmonary vascular resistance; Qpi, indexed pulmonary blood flow; Qsi, indexed systemic blood flow; sAP, systolic aortic pressure; sPAP, systolic pulmonary artery pressure; SVRI, indexed systemic vascular resistance.
Figure 3.

Percentage change of hemodynamic variables from baseline for iNO and ivS—non‐CHD group. The X‐axis shows percentage changes in hemodynamics variables for iNO and ivS from baseline, with the negative value showing reduction and positive value increment. Y‐axis shows hemodynamic variables on the left‐hand side and p values for the percentage changes between iNO and ivS on the right‐hand side. CHD, congenital heart disease; dAP, diastolic aortic pressure; dPAP, diastolic pulmonary artery pressure; iNO, inhaled nitric oxide; ivS, intravenous sildenafil; mAP, mean aortic pressure; mLA, mean left atrial pressure; mPAP, mean pulmonary artery pressure; mRA, mean right atrial pressure; PACI, indexed pulmonary artery capacitance; PVRI, indexed pulmonary vascular resistance; Qpi, indexed pulmonary blood flow; Qsi, indexed systemic blood flow; sAP, systolic aortic pressure; sPAP, systolic pulmonary artery pressure; SVRI, indexed systemic vascular resistance.
Figure 4.
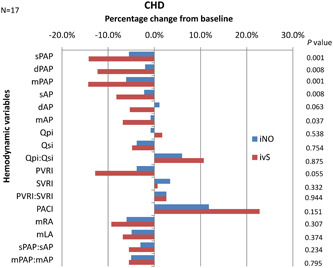
Percentage change of hemodynamic variables from baseline for iNO and ivS—CHD group. X‐axis shows percentage changes in hemodynamic variables for iNO and ivS from baseline, with the negative value showing reduction and positive value increment. Y‐axis shows hemodynamic variables on the left‐hand side and p values for the percentage changes between iNO and ivS on the right‐hand side. CHD, congenital heart disease; dAP, diastolic aortic pressure; dPAP, diastolic pulmonary artery pressure; iNO, inhaled nitric oxide; ivS, intravenous sildenafil; mAP, mean aortic pressure; mLA, mean left atrial pressure; mPAP, mean pulmonary artery pressure; mRA, mean right atrial pressure; PACI, indexed pulmonary artery capacitance; PVRI, indexed pulmonary vascular resistance; Qpi, indexed pulmonary blood flow; Qsi, indexed systemic blood flow; sAP, systolic aortic pressure; sPAP, systolic pulmonary artery pressure; SVRI, indexed systemic vascular resistance.
DISCUSSION
The present study demonstrates the excellent comparability of pulmonary and systemic hemodynamic changes between ivS and iNO during AVT. The study also describes the utility of ivS in the identification of acute responders when compared to iNO for the initiation of calcium channel blocker (CCB) in patients with IPAH.
The concept of AVT and acute responder evolved from much‐improved survival noted in IPAH patients who had an excellent vasodilatory response to high‐dose CCB. 23 Another common indication for AVT is for deciding operability in CHD with elevated PVR, especially in low resource environments where late presentation is quite common. 24 Though iNO remains the standard of care agent for AVT, there are vast regional variations in the agent of choice even in current era depending on availability, the experience of use and resource limitations and each agent may elicit a different pattern of hemodynamic response. 10 , 25 The ideal agent for AVT should be short‐acting, easy to administer, safe with predictable vasodilator responses, and inexpensive. Sildenafil is the most common pulmonary vasodilator used for the treatment of PAH with a proven safety profile across all age groups. 11 , 12 , 13 , 14 The ease and familiarity with the use and availability of intravenous formulation prompted us to consider ivS as a prospective agent for AVT.
There is a paucity of data regarding the usage of sildenafil for AVT. Milger et al. 26 analyzed retrospective data in their study of 198 adults on oral sildenafil (25 mg). The majority were females (63.6%) with two equal diagnostic subsets (99 each)—IPAH and associated PAH (APAH), the majority in the latter subset having connective tissue disorder (23.2%). Our study population had patients across all age groups with female predominance (61%), the commonest diagnosis being IPAH and operability assessment for CHD. Milger et al. 26 found that in the IPAH subset there was a comparable fall in mPAP from baseline with iNO and sildenafil (baseline: 51.6 ± 13.9, iNO: 44.1 ± 15.4, sildenafil: 44.6 ± 13.7 mmHg, p = 0.49). However, the fall in mean PVR (baseline 969 ± 458, iNO 789 ± 463, sildenafil 751 ± 403 dynes s cm−5, p < 0.001) and rise in mean cardiac index (baseline 2.1 ± 0.6, iNO 2.2 ± 0.6, sildenafil 2.4 ± 0.6 L/min/m2, p = 0.047) by sildenafil was statistically significant when compared to iNO. Sildenafil also resulted in a significant fall in mean RA pressures when compared to iNO. In our study, in non‐CHD group, ivS, when compared to iNO, produced a significant fall in mPAP and sAP, thus confirming the nonselective vasodilator property of sildenafil. The changes in all other hemodynamic variables were comparable, there was excellent ICC and the difference in the percentage change from baseline being ≤5.2% for all the variables. For the APAH subset in the study by Milger et al., 26 all the above‐mentioned variables decreased more with sildenafil with an increase in cardiac index, and all changes were statistically significant. Similarly, the CHD group in our study showed a predominant effect with ivS, there was excellent ICC and the difference in percentage change between the two agents was more pronounced (up to 11%) than in the non‐CHD group. In contrast to Milger et al.,. 26 we noticed a fall in cardiac output with both agents, the effect being comparable. The derived variables in our study were comparable for both the groups between two agents, possibly because the absolute differences in the measured variables were negated when converted to ratios.
Milger et al. 26 identified more acute responders (n = 23, 11.6%) with sildenafil when compared to iNO (n = 16, 8.1%) in the whole cohort, the difference more pronounced in the APAH subset (12.1% vs. 6.1%) and comparable in IPAH subset (11.1% vs. 10.1%). Among the IPAH subset, all the long‐term responders to CCB were identified by iNO and sildenafil, suggesting the utility of the latter in AVT in this subset. Overall, the 5‐year survival rate for the long‐term responders was comparable between the two agents. In our study, all acute responders had IPAH and were identified by both agents. We did not identify any responders in terms of operability in the CHD group with either agent thus mandating validation with further prospective studies. ivS was safe and well tolerated by our patients including children, while Milger et al. 26 reported systemic hypotension in 4.5% of adult patients responding to fluid resuscitation.
Day et al. 16 in a retrospective study (n = 13) among children studied the effect of iNO and ivS (median dose 0.16 mg/kg, range 0.05–0.29) with variable FiO2. There was a moderate positive correlation for mPAP and PVRI between iNO and ivS but a poor correlation with percentage changes from baseline and a significant lack of consistency in identifying responders.
iNO remains the standard of care agent for AVT due to its selective pulmonary vasodilator property. In comparison, ivS has the advantages of comparable hemodynamic properties with iNO, ease and familiarity of use, nonrequirement of complex delivery systems, widespread availability, and is safe. ivS over oral sildenafil has the benefit of quick action with peak plasma levels by 20 min compared to 30–60 min of the latter thereby reducing the testing time and more predictable bioavailability. To date, there are no prospective studies testing the utility of ivS as an agent for AVT in adults and children.
The study limitations include the use of the Ficks method and the LaFarge table for systemic and pulmonary blood flow calculations, which has inherent drawbacks. Flowmeter‐based iNO delivery has innate limitations since the actual inspired concentration can vary due to changes in breathing pattern, fresh gas flow rate into the circuit, and rebreathing. We did not measure the plasma levels of cGMP or sildenafil to demonstrate the effectiveness of drug administration. Moreover, though the acute responders were identified by both agents accurately, as expected the numbers were few, suggesting the necessity of large multicentre trials to validate the same.
To conclude, ivS could be a potential alternative agent for AVT in identifying acute responders for the initiation of CCB in children and adults. The hemodynamic effects of ivS on systemic and pulmonary circulation during AVT are comparable to iNO and is safe. The ease of use, familiarity, and easy availability could make it a potentially attractive option, especially in limited resource settings.
AUTHOR CONTRIBUTIONS
Substantial contributions to the conceptualization and design of the work, drafting the work and analysis, and final approval of the version: Shine Kumar. Substantial contributions to the acquisition of data for the work, preliminary manuscript writing, and final approval of the version: Danish Memon. Substantial contributions to the analysis and interpretation of data for the work, drafting and revising the work critically for important intellectual content, and final approval of the version: Manu Raj. Substantial contributions to the analysis of data for the work, creation of graphical content, and final approval of the version: Abish Sudhakar. Substantial contributions to the conceptualization and design of the work, acquisition of data for the work, and final approval of the version: Amitabh C. Sen. Substantial contributions to the acquisition of data for the work, preliminary manuscript writing, and final approval of the version: Jessin P. Jayasankar. Substantial contributions to the acquisition of data for the work, preliminary manuscript writing, and final approval of the version: Sreelakshmi P. Leeladharan. Substantial contributions to the conceptualization and design of the work, drafting and revising the work critically for important intellectual content, and final approval of the version: Raman K. Kumar.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Institutional Ethics Committee (ECASM‐AIMS‐21‐153). Written informed consent was obtained from all adult patients and from parents when within the pediatric age group.
ACKNOWLEDGMENTS
The authors sincerely acknowledge the critical review and suggestions by Dr Aveek Jayant, Former Professor and Head of the Department of Cardiac Anesthesia, Amrita Institute of Medical Sciences, Amrita Vishwa Vidyapeetham University, Kochi, Kerala, India. This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
Kumar S, Memon D, Raj M, Sen AC, Jayasankar JP, Leeladharan SP, Sudhakar A, Kumar RK. Comparison of intravenous sildenafil with inhaled nitric oxide for acute vasodilator testing in pulmonary arterial hypertension. Pulm Circ. 2022;e12180. 10.1002/pul2.12180
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.
REFERENCES
- 1. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raffy O, Azarian R, Brenot F, Parent F, Sitbon O, Petitpretz P, Hervé P, Duroux P, Dinh‐Xuan AT, Simonneau G. Clinical significance of the pulmonary vasodilator response during short‐term infusion of prostacyclin in primary pulmonary hypertension. Circulation. 1996;93(3):484–8. [DOI] [PubMed] [Google Scholar]
- 3. Barst RJ, Maislin G, Fishman AP. Vasodilator therapy for primary pulmonary hypertension in children. Circulation. 1999;99(9):1197–208. [DOI] [PubMed] [Google Scholar]
- 4. Douwes JM, van Loon RLE, Hoendermis ES, Vonk‐Noordegraaf A, Roofthooft MTR, Talsma MD, Hillege HL, Berger RMF. Acute pulmonary vasodilator response in paediatric and adult pulmonary arterial hypertension: occurrence and prognostic value when comparing three response criteria. Eur Heart J. 2011;32(24):3137–46. [DOI] [PubMed] [Google Scholar]
- 5. Sitbon O, Humbert M, Jagot J, Taravella O, Fartoukh M, Parent F, Herve P, Simonneau G. Inhaled nitric oxide as a screening agent for safely identifying responders to oral calcium‐channel blockers in primary pulmonary hypertension. Eur Respir J. 1998;12(2):265–70. [DOI] [PubMed] [Google Scholar]
- 6. Ricciardi MJ, Knight BP, Martinez FJ, Rubenfire M. Inhaled nitric oxide in primary pulmonary hypertension. J Am Coll Cardiol. 1998;32(4):1068–73. [DOI] [PubMed] [Google Scholar]
- 7. Atz AM, Adatia I, Lock JE, Wessel DL. Combined effects of nitric oxide and oxygen during acute pulmonary vasodilator testing. J Am Coll Cardiol. 1999;33(3):813–9. [DOI] [PubMed] [Google Scholar]
- 8. Tonelli AR, Alnuaimat H, Mubarak K. Pulmonary vasodilator testing and use of calcium channel blockers in pulmonary arterial hypertension. Respir Med. 2010;104(4):481–96. [DOI] [PubMed] [Google Scholar]
- 9. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 10. Sharma A, Obiagwu C, Mezue K, Garg A, Mukherjee D, Haythe J, Shetty V, Einstein AJ. Role of vasodilator testing in pulmonary hypertension. Prog Cardiovasc Dis. 2016;58(4):425–33. [DOI] [PubMed] [Google Scholar]
- 11. Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148–57. [DOI] [PubMed] [Google Scholar]
- 12. Rubin LJ, Badesch DB, Fleming TR, Galiè N, Simonneau G, Ghofrani HA, Oakes M, Layton G, Serdarevic‐Pehar M, McLaughlin VV, Barst RJ. Long‐term treatment with sildenafil citrate in pulmonary arterial hypertension. Chest. 2011;140(5):1274–83. [DOI] [PubMed] [Google Scholar]
- 13. Schulze‐Neick I, Hartenstein P, Li J, Stiller B, Nagdyman N, Hübler M, Butrous G, Petros A, Lange P, Redington AN. Intravenous sildenafil is a potent pulmonary vasodilator in children with congenital heart disease. Circulation. 2003;108(Suppl 1):II167–73. [DOI] [PubMed] [Google Scholar]
- 14. Dodgen AL, Hill KD. Safety and tolerability considerations in the use of sildenafil for children with pulmonary arterial hypertension. Drug Healthc Patient Saf. 2015;7:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document G. ESC Scientific Document Group 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119. [DOI] [PubMed] [Google Scholar]
- 16. Day RW. Comparison between the acute pulmonary vascular effects of oxygen with nitric oxide and sildenafil. Front Pediatr. 2015;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sharma V, Joshi S, Joshi A, Kumar G, Arora H, Garg A. Does intravenous sildenafil clinically ameliorate pulmonary hypertension during perioperative management of congenital heart diseases in children? ‐ A prospective randomized study. Ann Card Anaesth. 2015;18(4):510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stocker C, Penny DJ, Brizard CP, Cochrane AD, Soto R, Shekerdemian LS. Intravenous sildenafil and inhaled nitric oxide: a randomised trial in infants after cardiac surgery. Intensive Care Med. 2003;29(11):1996–2003. [DOI] [PubMed] [Google Scholar]
- 19. Hill KD, Sampson MR, Li JS, Tunks RD, Schulman SR, Cohen‐Wolkowiez M. Pharmacokinetics of intravenous sildenafil in children with palliated single ventricle heart defects: effect of elevated hepatic pressures. Cardiol Young. 2016;26(2):354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sitbon O, Humbert M, Jaïs X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Hervé P, Simonneau G. Long‐term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111(23):3105–11. [DOI] [PubMed] [Google Scholar]
- 21. Douwes JM, Humpl T, Bonnet D, Beghetti M, Ivy DD, Berger RMF, Weintraub RG, Geiger R, Marx M, Jing ZC, Sondergaard L, Apitz C, Hager A, Szatmari A, Milanesi O, Saji T, Pulido T, Moll J, Michalak KW, Kawalec W, Zuk M, Fasnacht Boillat M, Olguntürk R, Kula S, Alehan D, Schulze‐Neick I, Atz AM, Mallory GB, Austin ED, Moore DJ, Feinstein JA, Day RW, Yung D, Berger JT. Acute vasodilator response in pediatric pulmonary arterial hypertension. J Am Coll Cardiol. 2016;67:1312–23. [DOI] [PubMed] [Google Scholar]
- 22. Cerro MJ, Moledina S, Haworth SG, Ivy D, Dabbagh MA, Banjar H, Diaz G, Heath‐Freudenthal A, Galal AN, Humpl T, Kulkarni S, Lopes A, Mocumbi AO, Puri GD, Rossouw B, Harikrishnan S, Saxena A, Udo P, Caicedo L, Tamimi O, Adatia I. Cardiac catheterization in children with pulmonary hypertensive vascular disease: consensus statement from the Pulmonary Vascular Research Institute, Pediatric and Congenital Heart Disease Task Forces. Pulm Circ. 2016;6(1):118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rich S, Brundage BH. High‐dose calcium channel‐blocking therapy for primary pulmonary hypertension: evidence for long‐term reduction in pulmonary arterial pressure and regression of right ventricular hypertrophy. Circulation. 1987;76(1):135–41. [DOI] [PubMed] [Google Scholar]
- 24. Rashid U, Qureshi A, Hyder S, Sadiq M. Pattern of congenital heart disease in a developing country tertiary care center: factors associated with delayed diagnosis. Ann Pediatr Cardiol. 2016;9(3):210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caicedo L, Hopper R, Garcia Aguilar H, Ivy D, Haag D, Fineman J, Humpl T, Al‐Tamimi O, Feinstein JA, Berger R, Rosenzweig E, Kashour T, Diaz GF, Mendoza A, Krishnan U, Bobhate P, Handler S, Lopes AA, Kumar Rahit M, Barward P, Labrandero de Lera C, Adatia I, Moledina S, Abman S, Cerro MJ. Acute vasoreactivity testing in pediatric idiopathic pulmonary arterial hypertension: an International Survey on Current Practice. Pulm Circ. 2019;9(4):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Milger K, Felix JF, Voswinckel R, Sommer N, Franco OH, Grimminger F, Reichenberger F, Seeger W, Ghofrani HA, Gall H. Sildenafil versus nitric oxide for acute vasodilator testing in pulmonary arterial hypertension. Pulm Circ. 2015;5(2):305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.


