Abstract
Background:
Patients with renal impairment (RI) are typically excluded from trials evaluating chimeric antigen receptor (CAR) T-cell therapies. We evaluated the outcomes of patients with RI receiving standard-of-care (SOC) CAR T-cell therapy for relapsed/refractory (R/R) diffuse large B cell lymphoma (DLBCL).
Patients and Methods:
This was a retrospective, single-center cohort study of R/R DLBCL patients treated with SOC axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel) after 2 or more prior lines of therapy. Renal and survival outcomes were compared based on RI and fludarabine (flu) dose-reduction (DR) status. RI was defined by an estimated glomerular filtration rate of <60 mL/min/1.73 m2 as determined by the Modification of Diet in Renal Disease equation using day −5 creatinine (Cr) values. Acute kidney injury (AKI) was identified and graded using standard Kidney Disease: Improving Global Outcomes criteria. Renal recovery occurred if Cr was within 0.2 mg/mL of baseline by day +30. Flu was considered DR if given at <90% of the recommended FDA label dose.
Results:
Among 166 patients treated with CAR T-cell therapy there were 17 (10.2%) patients with baseline RI and 149 (89.8%) without RI. After CAR T-cell infusion, the incidence of any grade AKI was not significantly different between patients with baseline RI compared to those without RI (42% vs. 21% p=0.08). Similarly, severe grade 2/3 AKIs were seen in 1/17 (5.8%) with baseline RI and 11/149 (7.3%) without RI (p=1). Decreased renal perfusion (28/39, 72%) was the most common cause of AKI with cytokine release syndrome (CRS) contributing to 17/39 AKIs (44%). Progression-free-survival (PFS) and overall survival (OS) did not differ between patients with or without RI or between those with standard-dosed or dose-reduced fludarabine. By contrast, patients experiencing AKI had worse clinical outcomes than those without AKI (multivariable PFS HR 2.1, 95%CI 1.2–3.7; OS HR 3.9, 95%CI 2.1–7.4). Notably, peak inflammatory cytokine levels were higher in patients who experienced AKI. Finally, we describe two patients with end-stage renal disease (ESRD) on dialysis who received lymphodepletion and CAR T-cell therapy.
Conclusion:
Baseline renal function did not affect renal or efficacy outcomes after CAR T-cell therapy in DLBCL. On the other hand, patients with AKIs went on to experience worse clinical outcomes. AKIs were commonly related to CRS and high peak inflammatory cytokine levels. CAR T-cell therapy is feasible in ESRD patients and requires careful planning of lymphodepletion.
Introduction:
Chimeric antigen receptor (CAR) T-cell therapy has changed the therapeutic landscape of various B-cell non-Hodgkin lymphoma (NHL) subtypes. Pivotal trials have led to the approval of three CAR T-cell products for the treatment of relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) that has progressed following two lines of chemoimmunotherapy (CIT): axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisacel), and lisocabtagene maraleucel (liso-cel).1–3 Subsequently, axi-cel and liso-cel have received additional FDA approvals for second-line treatment of patients with R/R DLBCL relapsing within 12 months of frontline CIT.4,5 In addition, liso-cel has been approved for R/R LBCL at any time after first-line chemoimmunotherapy in patients who are ineligible for ASCT on the basis of age or comorbidity.6
Patients undergo comprehensive vital organ testing prior to infusion of these CAR T-cell products. Chronic kidney disease (CKD) is commonly encountered during this pretreatment evaluation. It is estimated that 10–20% of cancer patients receiving therapy have stage III CKD or worse, which is defined by an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2.7 Renal dysfunction not only implies frailty that is accounted for in the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI), but it also has implications for the use of lymphodepletion prior to CAR T-cell infusion.8,9 Fludarabine is a key component of lymphodepletion whose major metabolite, 2-F-ara-A, is primarily excreted in the urine. Patients with a creatinine clearance (CrCl) less than 60 mL/min are recommended to receive dose-reductions (DR) of fludarabine to prevent toxicities, such as prolonged myelosuppression and neurotoxicity.10 Yet, at the same time, there is concern that reducing fludarabine dose may adversely affect treatment efficacy.11,12 Patients with renal impairment (RI) are typically excluded from trials evaluating chimeric antigen receptor CAR T-cell therapies. As a result, there is a paucity of data available examining outcomes in this group of patients. We evaluated the renal and oncologic outcomes of patients with RI receiving SOC anti-CD19 directed CAR T-cell therapy for R/R DLBCL at our institution. We also describe anti-CD19 directed CAR T-cell therapy in two dialysis-dependent end-stage renal disease (ESRD) lymphoma patients.
Patients and Methods
This is a retrospective, single-center cohort study of R/R DLBCL patients after two or more prior lines of therapy treated with SOC axi-cel or tisa-cel as of 3/4/2021. We also case report a patient with Mantle Cell lymphoma who had baseline dialysis dependence and received brexucabtagene autoleucel (brexu-cel). For the DLBCL patients, renal and survival outcomes were compared based on RI and fludarabine DR status. RI was defined by an eGFR of <60 mL/min/1.73 m2 by the Modification of Diet in Renal Disease (MDRD) equation using day −5 creatinine (Cr) at the start of lymphodepletion. CrCl was calculated with the Cockcroft-Gault equation using day −5 serum creatinine and actual/adjusted body weights as appropriate. Acute kidney injury (AKI) identification and grading were assessed using Kidney Disease: Improving Global Outcomes (KDIGO) criteria. An onconephrologist (CB) reviewed all cases of AKI and assigned them into one of three categories that best describes the cause of the AKI: 1) decreased renal perfusion, including cytokine release syndrome (CRS), dehydration, hypotension, etc., 2) intrinsic renal injury, including acute tubular necrosis (ATN) from any etiology, and 3) obstructive nephropathy. Renal recovery occurred if Cr was within 0.2 mg/dL of baseline (BL) by day +30, in line with the Acute Disease Quality Initiative Workgroup 16 consensus definition of renal recovery.13 Categorical data were compared using Chisquare and Fisher’s exact tests. Statistical significance for continuous data was tested using a two samples t-test for mean values and the Mann-Whitney U-test for distribution/median values. Kaplan-Meier survival curves and log-rank tests were used to compare progression-free survival (PFS) and overall survival (OS) starting from the date of CAR T-cell infusion. The predictors of OS and PFS were investigated to report hazard ratios (HRs) and 95% confidence intervals (95% CIs) using AKI as the time-dependent variable by adjusting for gender, double/triple-hit status by fluorescence in situ hybridization (FISH), and the International Prognostic Index (IPI) score in the Cox proportional hazards regression analysis with a p-value of <0.05 deemed statistically significant. Blood samples were collected from patients throughout the process of CAR T-cell therapy for cytokine levels as previously described.14,15 This study was conducted under Advarra IRB Pro00024557.
For illustrative purposes, two patients who received lymphdepletion chemotherapy while on dialysis are described in more detail. Patient 1 is included in the cohort described above and is a 64F with R/R DLBCL who was dialysis-dependent after experiencing platinum nephrotoxicity from second-line R-ICE chemotherapy. Patient 2 is a 74M with p53-mutated R/R Mantle Cell lymphoma (MCL) and his outcomes are not reported as part of the above cohort. Prior to his MCL diagnosis he was dialysis-dependent following renal cell carcinoma treated with bilateral nephrectomy (partial on one side).
Results
Baseline Characteristics
One hundred sixty-six patients were included in the analysis with a median age of 65 years. Twenty-two patients (13%) received tisa-cel, while 144 (87%) received axi-cel. Fifty-six percent were male, and 44% were female. Most patients (81%) had advanced disease and high-intermediate or high IPI scores (58%). At the time of leukapheresis for CAR T-cell therapy, 16% had bulky disease > 10 cm, and 14% had an ECOG performance status of ≥ 2. RI at day −5 was present in 17/166 (10%) patients --- sixteen stage III and one ESRD patient on dialysis. Bulky disease was more commonly present in patients with RI (40% in RI vs. 14% in non-RI; p=0.019). Otherwise, age, gender, stage of disease, IPI score, ECOG score, and CAR T-cell products used were similar between the RI and non-RI groups. (Table 1).
Table 1. Patient characteristics and outcomes for patients according to baseline renal impairment or development of subsequent acute kidney injury.
Statistical significance (p<0.05) is denoted by shaded orange rows. Numbers represent mean values unless otherwise specified in the table. AKI: acute kidney injury, CRP: C-reactive protein, CRRT: continuous renal replacement therapy, CRS: cytokine release syndrome, ECOG: Eastern Cooperative Oncology Group, eGFR: estimated glomerular filtration rate, GCSF: granulocyte-colony stimulating factor, ICANS: immune effector cell-associated neurotoxicity syndrome, ICU: intensive care unit, IPI: International Prognostic Index, LDH: lactate dehydrogenase.
| Baseline Renal Impairment | Acute Kidney Injury During CAR-T | |||||
|---|---|---|---|---|---|---|
| Baseline Characteristics | Yes | No | p-value | Yes | No | p-value |
| Male; Female |
6/17 (35%); 11/17 (65%) |
87/149 (58%); 62/149 (42%) |
0.077 |
23/39 (59%); 16/39 (41%) |
70/127 (55%); 57/127 (45%) |
0.715 |
| White; Black; Asian; Other |
13/17 (76%); 1/17 (6%); 2/17 (12%); 1/17 (6%) |
133/147 (90%); 6/147 (4%); 5/147 (3%); 3/147 (2%) |
0.182 |
33/39 (85%); 3/39 (8%); 2/39 (5%); 1/39 (3%) |
113/125 (91%); 4/125 (3%); 5/125 (4%); 3/125 (2%) |
0.791 |
| Axi-cel; Tisa-cel |
14/17 (82%); 3/17 (18%) |
130/149 (87%); 19/149 (13%) |
0.703 |
36/39 (92%); 3/39 (8%) |
108/127 (85%); 19/127 (15%) |
0.293 |
| Median Age | 65.0 years | 65.0 years | 0.834 | 65.0 years | 64.9 years | 0.982 |
| Stage III/IV | 16/17 (94%) | 118/149 (79%) | 0.199 | 32/39 (82%) | 100/127 (79%) | 0.821 |
| ECOG ≥ 2 | 4/17 (24%) | 19/149 (13%) | 0.216 | 6/39 (15%) | 17/127 (14%) | 0.792 |
| IPI ≥ 3 | 12/17 (71%) | 80/143 (56%) | 0.306 | 27/39 (69%) | 65/121 (54%) | 0.097 |
| Bulky Disease | 6/15 (40%) | 20/145 (14%) | 0.019 | 10/38 (26%) | 16/122 (13%) | 0.076 |
| Double Hit by FISH | 3/14 (21%) | 27/104 (26%) | 1.000 | 6/28 (21%) | 22/93 (24%) | 1.000 |
| Baseline eGFR (mL/min/1.73m2) | 45.1 +/− 13.2 | 95.2 +/− 30.4 | <0.001 | 86.7 +/− 38.0 | 91.1 +/− 31.2 | 0.462 |
| Baseline LDH (U/L) | 315.1 +/− 227.7 | 379.2 +/− 324.1 | 0.431 | 496.7 +/− 520.4 | 332.9 +/−201.9 | 0.073 |
| Basline Ferritin (ng/mL) | 594.6 +/− 596.6 | 697.4 +/− 833.0 | 0.656 | 922.7 +/− 1231.6 | 610.7 +/− 608.4 | 0.198 |
| Baseline CRP (mg/dL) | 3.4 +/− 6.0 | 2.4 +/− 3.5 | 0.363 | 3.2 +/− 4.6 | 2.3 +/− 3.6 | 0.267 |
| Baseline Na (mmol/L) | 137.7 +/− 3.8 | 138.2 +/− 3.4 | 0.598 | 137.2 +/− 4.1 | 138.4 +/− 3.1 | 0.111 |
| Baseline Uric Acid (mg/dL) | 4.7 +/− 2.3 | 4.4 +/− 1.5 | 0.59 | 4.5 +/− 2.0 | 4.4 +/− 1.5 | 0.789 |
| Post CAR-T Characteristics | ||||||
| Peak CRP (mg/dL) | 19.0 +/− 14.8 | 14.3 +/− 9.7 | 0.079 | 20.2 +/− 12.5 | 13.2 +/− 9.1 | <0.001 |
| Na Nadir (mmol/L) | 132.4 +/− 3.8 | 131.3 +/− 4.0 | 0.291 | 130.2 +/− 4.0 | 131.8 +/− 3.9 | 0.021 |
| Day +7 Uric Acid (mg/dL) | 2.5 +/− 1.0 | 2.4 +/− 1.1 | 0.902 | 2.7 +/− 1.4 | 2.3 +/− 1.0 | 0.157 |
| Max CRS Grade | 1.4 +/− 1.1 | 1.5 +/− 0.9 | 0.623 | 2.1 +/− 1.2 | 1.3 +/− 0.7 | <0.001 |
| CRS ≥ Grade 3 | 2/17 (12%) | 12/149 (8%) | 0.639 | 9/39 (23%) | 5/127 (4%) | <0.001 |
| Max ICANS Grade | 1.7 +/− 1.6 | 1.3 +/− 1.3 | 0.317 | 1.9 +/− 1.5 | 1.2 +/− 1.3 | 0.005 |
| ICANS ≥ Grade 3 | 6/17 (35%) | 35/149 (23%) | 0.372 | 14/39 (36%) | 27/127 (21%) | 0.088 |
| Tocilizumab Use | 9/17 (53%) | 78/149 (52%) | 1 | 29/39 (74%) | 58/127 (46%) | 0.002 |
| Steroid Use | 9/17 (53%) | 71/149 (48%) | 0.8 | 24/39 (62%) | 56/127 (44%) | 0.068 |
| Vancomycin Use | 4/17 (24%) | 31/149 (21%) | 0.759 | 13/39 (33%) | 22/127 (17%) | 0.043 |
| Acute Kidney Injury | 7/17 (41%) | 32/149 (21%) | 0.078 | 39 | 127 | N/A |
| Need for CRRT | 0/7 (0%) | 5/32 (16%) | 0.563 | 6/39 (15%) | N/A | N/A |
| Renal Recovery After AKI | 5/7 (71%) | 22/32 (69% | 1 | 27/39 (69%) | N/A | N/A |
| ICU admission | 3/17 (18%) | 22/149 (15%) | 1 | 13/39 (33%) | 12/127 (9%) | <0.001 |
| Receipt of IVIG | 4/17 (24%) | 25/147 (17%) | 0.506 | 6/38 (16%) | 23/126 (18%) | 0.813 |
| Receipt of GCSF | 11/17 (65%) | 56/147 (38%) | 0.093 | 17/38 (45%) | 50/126 (40%) | 0.578 |
Outcomes of Patients with Baseline Renal Impairment
At a median follow up of 13.0 months (mos.), median PFS (mPFS) was 8.9 mos. (95% CI:2.6–11.5) in RI vs 9.9 mos. (95% CI: 5.8–24.8) in non-RI patients (p=0.75, Figure 1A). Median overall survival (mOS) was 10.4 mos. (95% CI:4.9–11.5) in RI vs not reached (N.R.) in non-RI patients (p=0.14, Figure 1B).
Figure 1. Clinical outcomes in patients with renal impairment or fludarabine dose reduction.
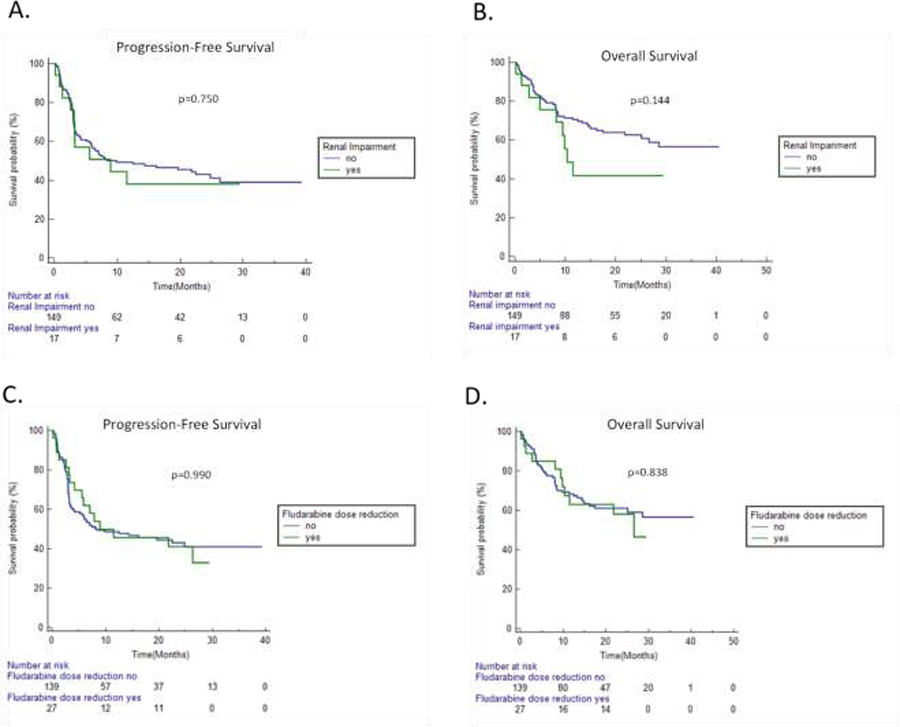
A. Progression-free survival (PFS) stratified by renal impairment (RI) at time of initiating lymphodepletion. B. Overall survival (OS) stratified by RI at time of initiating lymphodepletion. C. PFS stratified by fludarabine dose reduction (DR). D. OS stratified by fludarabine DR.
Baseline ferritin, C-reactive protein (CRP), and lactate dehydrogenase (LDH) were similar in RI and non-RI patients. Additionally, peak CRP, maximum CRS grade, maximum immune effector cell-associated neurotoxicity syndrome (ICANS) grade, tocilizumab use, steroid use, intravenous immune globulin (IVIG) use, and granulocyte-colony stimulating factor (G-CSF) use were similar regardless of RI status (Table 1).
Outcomes of Patients Receiving Fludarabine Dose-Reduction
Twenty-seven patients (16.3%) received DR fludarabine. Percentage fludarabine dose-reduction was <20% (N=2), 20–39% (N=23), and 40–50% (N=2). All patients were DR due to RI except for one patient who had a 10% dose-reduction to compensate for body surface area loss with prior limb amputation. Twenty-four of the 26 patients that received DR fludarabine for RI had a dosing CrCl of less than 60 mL/min. The other two patients were DR based on provider discretion for age and CrCl values close to 60 mL/min. Baseline patient and disease characteristics were similar in DR and standard-dosed (SD) fludarabine patients, except bulky disease was more commonly present in DR patients (33% vs. 13%; p=0.03, Supplementary Table S1). Median PFS was 8.9 mos. (95% CI:4.2–26.3) in DR vs. 8.4 mos. (95% CI:5.1–24.8) in SD fludarabine patients (p=0.99, Figure 1C). Median OS was 26.6 mos. (95% CI:9.8–26.6) in DR vs. N.R. in SD fludarabine patients (p=0.84, Figure 1D). No significant differences in peak CRP, maximum CRS/ICANS grade, and tocilizumab, steroid, IVIG, and G-CSF use were found between DR and SD fludarabine groups (Supplementary Table S1).
Acute Kidney Injury
In total for the 166 CAR T-cell treated patients, 39 (23%) had an AKI between days −5 and 30. Five AKIs (13%) occurred between days −5 and 0, while the remaining 34 (87%) occurred after CAR T-cell infusion. In the majority of cases, the AKI was grade 1 (69% of all AKI), but grade 2 (13%) and grade 3 AKIs also occurred (18%). Decreased renal perfusion (28/39, 72%) was the most common cause of AKI with CRS contributing toward 17/39 AKIs (44%) overall. Intrinsic renal injury accounted for 10/39 AKIs (26%), while there was one case of obstructive nephropathy (3%). Tumor lysis syndrome (TLS) was not observed in any of our patients with AKI.
No differences in baseline patient or disease characteristics were observed between patients who subsequently developed AKI and those who did not (Table 1). Baseline RI did not associate with a higher propensity for AKI. Any AKI occurred in 7/17 with baseline RI (42%) and 32/149 without baseline RI (21%) (p=0.08). Grade 2/3 AKIs were seen in 1/17 (6%) RI and 11/49 (7%) non-RI patients (p=1). However, differences were noted in the post-CAR T cell infusion period for patients with AKI, consistent with renal effects and concomitant other toxicities. Patients with AKI had a lower sodium nadir (130.2 +/− 4.0 vs. 131.8 +/− 3.9; p=0.021), greater vancomycin use (33% vs. 17%; p=0.043) and more ICU admissions (33% vs. 9%; p<0.001; Table 1). Complete renal recovery by day +30 after AKI was seen in 5/7 (71%) RI and 22/32 (69%) non-RI patients (p=1). The majority of those without complete renal recovery had expired before day +30 (7/12, 58%). Four of these patients died from lymphoma progression, two died from CAR T-cell therapy toxicity (CRS/ICANS), and one died from pneumonia. Of the remaining five who did not expire before day +30, one died at day +37 from hemophagocytic lymphohistiocytosis16, two eventually had complete renal recovery to prior baseline creatinine, and the other two had new baseline creatinine values 0.3 mg/dL higher than their pre-CAR baseline. Four of the five patients who required continuous renal replacement therapy expired prior to day +30. Two of the patients died from lymphoma progression, while the other two as a result of CRS. The fifth patient developed anuric renal failure from rhabdomyolysis as a consequence of CRS within 1 week after CAR T-cell infusion, remained on hemodialysis for 42 days, and then recovered by day +90 to an unsupported eGFR greater than 60 mL/min/m2.
Overall clinical outcomes were worse in patients with any grade AKI compared to those without AKI. Median PFS was 3.2 mos. (95% CI:1.3–7.4) in AKI vs. 19.6 mos. (95% CI: 7.1–26.3) in non-AKI patients (p=0.003, Figure 2A). Median OS was 9.8 mos. (95% CI:3.4–16.2) in AKI vs. not reached in non-AKI patients (p<0.001, Figure 2B). The worst clinical outcomes occurred in patients with severe AKI. By grade, the median PFS was 0.8 mos. (95% CI:0.3–2.1) in G2/3 AKI patients vs. 6.0 mos. (95% CI:3.0–16.2) in G1 AKI patients (p<0.001, Figure 2C). The median OS was 0.9 mos. (95% CI:0.3–3.4) in G2/3 AKI patients vs. 14.6 mos. (95% CI:9.4–16.2) in G1 AKI patients (p<0.001, Figure 2D). We then performed multivariate Cox regression analysis, including the development of AKI with baseline variables known to affect DLBCL outcome. Patients who developed any grade AKI had significantly worse PFS (HR 2.1; 95% CI:1.2–3.7) and OS (HR 3.9; 95% CI:2.1–7.4) (Table 2). The presence of baseline RI did not appear to impact the effect of an AKI on outcomes. There was no statistical difference in median PFS (3.2 mos. for both groups, p=0.45) or median OS (9.8 mos. for both groups, p=0.39) for patients who had an AKI during CAR T-cell therapy when stratified by RI at time of lymphodepletion (Supplementary Figures S1A and S1B).
Figure 2. Clinical outcomes in patients experiencing AKI.
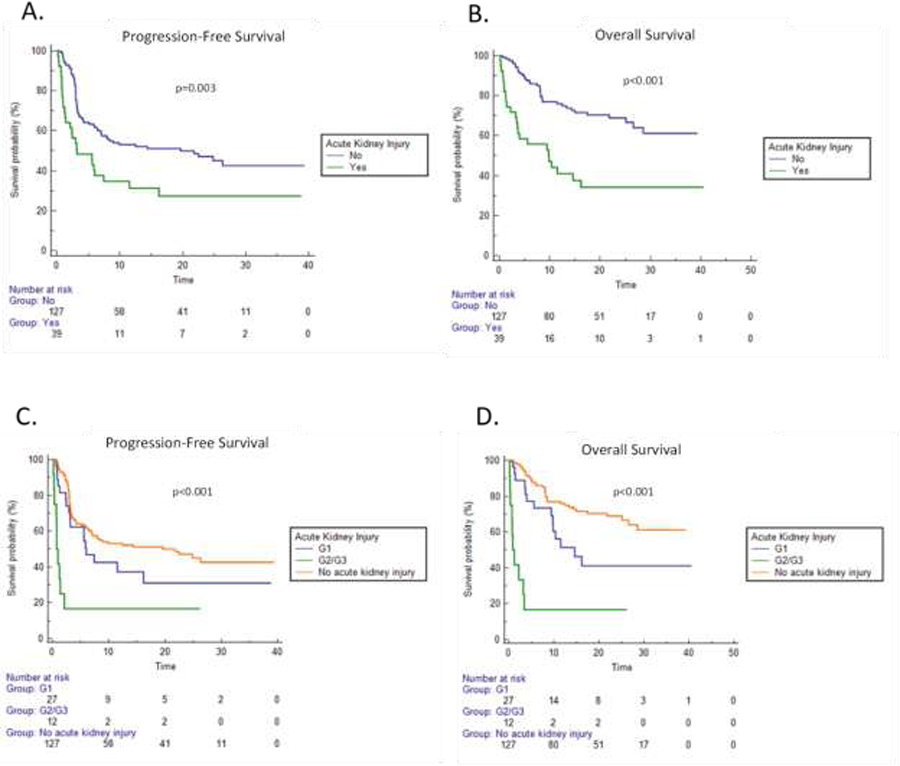
A. Progression-free survival (PFS) stratified by presence or lack thereof an acute kidney injury (AKI). B. Overall survival (OS) stratified by presence or lack thereof an AKI. C. PFS stratified by severity (Grade = G) of AKI. D. OS stratified by severity of AKI.
Table 2. Multivariate Analysis for PFS and OS.
Cox proportional hazards regression analysis reporting hazard ratios (HRs) and 95% confidence intervals (95% CIs) using acute kidney injury (AKI) as the time-dependent variable by adjusting for gender, double/triple-hit status by fluorescence in situ hybridization (FISH), and the International Prognostic Index (IPI) score. Statistical significance (p<0.05) is denoted by shaded orange rows.
| Progression Free Survival | ||||
|---|---|---|---|---|
| Factor | Univariate Cox Regression Hazard Ratio (95% CI) |
p-value | Multivariate Cox Regression Hazard Ratio (95% CI) |
p-value |
| Presence of AKI | 1.91 (1.21 – 3.01) | 0.005 | 1.99 (1.16 – 3.40) | 0.012 |
| Male Gender | 1.47 (0.97 – 2.23) | 0.071 | 1.68 (1.03 – 2.71) | 0.036 |
| Double or Triple-hit by FISH | 1.19 (0.71 – 1.99) | 0.516 | 1.04 (0.62 – 1.75) | 0.888 |
| IPI Score | 1.67 (1.36 – 2.06) | <0.001 | 1.77 (1.41 – 2.22) | <0.001 |
| Overall Survival | ||||
| Factor | Univariate Cox Regression Hazard Ratio (95% CI) |
p-value | Multivariate Cox Regression Hazard Ratio (95% CI) |
p-value |
| Presence of AKI | 3.08 (1.84 – 5.15) | <0.001 | 3.52 (1.96 – 6.35) | <0.001 |
| Male Gender | 1.97 (1.13 – 3.41) | 0.016 | 2.83 (1.52 – 5.25) | 0.001 |
| Double or Triple-hit by FISH | 1.35 (0.75 – 2.44) | 0.312 | 1.05 (0.58 – 1.90) | 0.879 |
| IPI Score | 1.98 (1.53 – 2.57) | <0.001 | 2.23 (1.67 – 2.96) | <0.001 |
CRS and cytokines
Consistent with CRS being commonly seen during AKIs, patients who developed AKI after CAR T-cell therapy had greater peak CRP (20.2 +/− 12.5 vs. 13.2 +/− 9.1; p<0.001), a higher incidence of grade 3 or higher CRS (23% vs. 4%; p<0.001), and more tocilizumab use (74% vs. 46%; p=0.002) when compared with non-AKI patients (Table 1). There were no statistical differences in baseline day −6 or day 0 cytokine levels between patients with or without subsequent AKI (Supplementary Figures S2 and S3). However, peak IL-2 (47.7 +/− 39.7 vs. 21.0 +/− 24.8; p=0.006), IL-6 (7317.5 +/− 11,522.3 vs. 1568.0 +/− 3380.7; p=0.031), TNF-alpha (38.5 +/− 26.9 vs. 23.2 +/− 15.8; p=0.017), and ANG-2/ANG-1 (4.0 +/− 6.1 vs. 0.7 +/− 1.0; p=0.019) values were all higher in AKI vs. non-AKI patients (Figure 3). Meanwhile, IL-1beta, IL-15, IFN-gamma, and GM-CSF levels were statistically similar between AKI and non-AKI patients. There were no differences in baseline or peak cytokine levels in patients with or without RI. Furthermore, there were no differences in peak or day 0 cytokine levels based on fludarabine dose-reduction status (Supplementary Figures S4 and S5).
Figure 3. Peak cytokine levels after CAR T cell therapy are higher in patients who developed AKI.
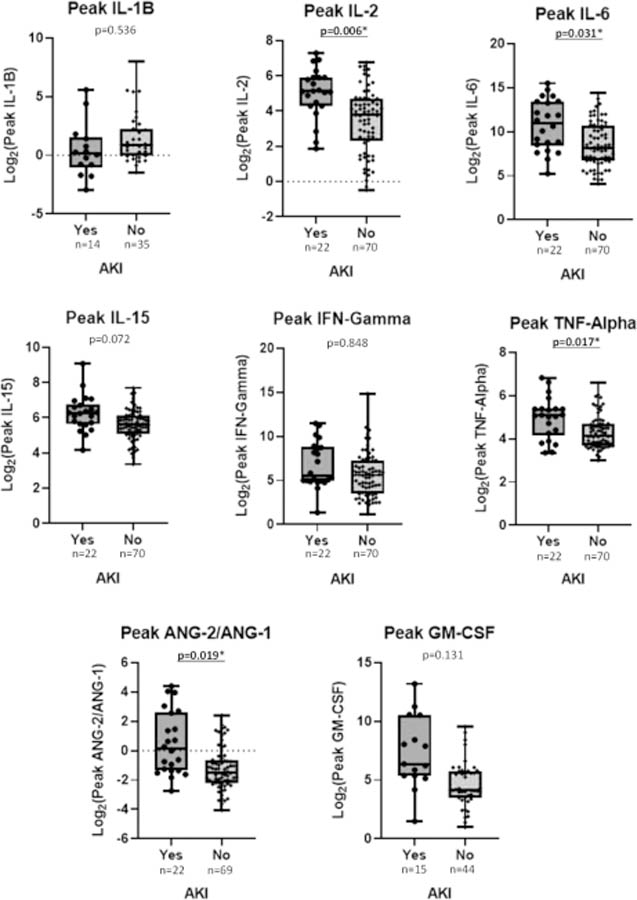
Peak cytokine levels (y-axis) stratified by presence or lack of AKI (x-axis) between days −5 and +30 during CAR T-cell therapy. Underlined and asterisked p-values denote statistical significance (p<0.05 by T-test).
CAR T cell therapy in patients with ESRD
As part of the above cohort, one patient (Patient 1) had baseline dialysis-dependent ESRD. Her dialysis and lymphodepletion dosing schedules are shown in Supplementary Figure S6A. On day 0, she received tisa-cel infusion. Her post CAR T course was complicated by neutropenic fever, atrial fibrillation, colitis, G1 CRS, and G4 ICANS that resolved with corticosteroid treatment. Her day +30 CT scan demonstrated a complete response (CR). She remained in remission until a PET/CT scan on Day + 271 showed widespread extranodal recurrence of her disease in the lungs, liver, bones, and musculature. For illustrative purposes we also describe a second dialysis-dependent ESRD patient (Patient 2), who was not part of the DLBCL cohort above, and had Mantle Cell Lymphoma (MCL) treated with brexucabtagene autoleucel (brexu-cel). His dialysis and lymphodepletion dosing schedules are shown in Supplementary Figure S6B. During lymphodepletion he required sevelamer for mild hyperphosphatemia. His post CAR T course was complicated by hypertension requiring hydralazine, G2 CRS, and volume overload requiring loop diuretics. His day +30 PET/CT showed a partial response (PR), but on Day +90 follow-up imaging showed disease progression.
Discussion
In this study we found that patients with RI at the start of lymphodepletion have similar renal and CAR T-cell outcomes compared to patients without RI. However, patients with an AKI between days −5 and +30 had decreased PFS and OS as compared to those who did not experience an AKI. CRS was a common cause of AKI in CAR T-cell patients, with patients who experienced an AKI having higher grade CRS and higher peak cytokine levels. We demonstrated that it is feasible to administer CAR T-cell products in lymphoma patients who are dialysis-dependent at baseline with careful planning of lymphodepletion.
Previously, AKIs were observed in a single-institution retrospective review of 46 patients receiving axi-cel and tisa-cel for the management of R/R DLBCL.17 The results were similar to that reported here, with frequent findings of any grade AKI (23%), G2/G3 AKIs (30% of AKIs), and renal recovery following AKI (71%). Another study reported on 78 patients from two institutions receiving axi-cel and tisa-cel for the management of R/R DLBCL.18 Again, overall rates of AKI were similar (19%), and a proportion of patients required RRT. However, only two patients in that study had a baseline eGFR of less than 60 mL/min/m2, both of whom developed an AKI. In our study of 166 patients, 17 patients had a baseline eGFR of less than 60 ml/min/m2. We did not find a statistically significant association between the propensity to develop AKIs in patients with baseline RI (42% vs. 21%; p=0.08). Moreover, most AKIs in patients with RI was of minor severity (86% grade 1), and patients with RI had rates of renal recovery similar to that of patients without RI. Therefore, RI at the start of lymphodepletion does not seem clinically relevant on overall renal outcome despite a possible propensity to develop mild AKIs.
Lymphodepletion is important to allow for proper expansion and survival of CAR T-cell products. Fludarabine and cyclophosphamide are the most common lymphodepleting agents. Fludarabine’s major metabolite, 2-F-ara-A, is primarily excreted in the urine. The prior studies on renal dysfunction and CAR T cell therapy in DLBCL did not address fludarabine dose reduction.17,18 However, fludarabine dose intensity before CAR T-cell therapy has been associated with survival outcomes in two recent studies in pediatric B-ALL.13,19 In our study, fludarabine dose reduction for RI did not affect survival outcomes. This may be because the dose reduction in these patients was counterbalanced by decreased excretion, resulting in similar fludarabine exposure. Indeed, we did not find differences based on fludarabine dose-reduction for key cytokine levels such as IL-15. More research is needed to define the optimal dose of fludarabine in CAR T-cell patients and the effect of renal impairment. Emblematic of the lack of clarity on optimal dosing, the standard dose of fludarabine and cyclophosphamide differs across FDA-approved CAR T-cell products for DLBCL: tisa-cel (fludarabine 25 mg/m2; cyclophosphamide 250 mg/m2), axi-cel (fludarabine 30 mg/m2; cyclophosphamide 500 mg/m2), and liso-cel (fludarabine 30 mg/m2; cyclophosphamide 300 mg/m2).
As fludarabine is only 19–29% protein bound, it has previously been demonstrated that it can be adequately dialyzed in patients with ESRD.11,12 Cyclophosphamide has similar protein-binding characteristics with a potential to be dialyzed as well, albeit it is primarily hepatically metabolized with only 10–20% of unchanged drug being excreted into the urine.20,21 We illustrated our approach to lymphodepletion in two patients with ESRD in Supplementary Figure S6. Patient 1 received tisa-cel for the management of R/R DLBCL. The standard lymphodepletion regimen for tisa-cel is fludarabine (25 mg/m2) and cyclophosphamide (250 mg/m2). Extrapolating from the transplant literature, we elected to dose-reduce fludarabine by 50% to 12.5 mg/m2.22,23 To compensate for this reduction in fludarabine in which we risked suboptimal lymphodepletion, the cyclophosphamide dose was increased to 500 mg/m2. Dialysis was performed on day −5 and day −3, approximately 12 hours following chemotherapy. Tisa-cel was given to the patient as soon as possible after dialysis was completed on day 0. Patient 1 experienced severe ICANS (grade 4), which is somewhat unusual for patients treated with tisa-cel. We considered fludarabine neurotoxicity in our differential diagnosis but her course was consistent with ICANS as it began on day +5, and completely resolved with corticosteroid therapy within 72 hours. It is possible that minor fludarabine toxicity may exacerbate ICANS, or that dialysis-dependent patients may experience more severe encephalopathy from any cause due to abnormal renal elimination. Patient 2 received brexu-cel for the management of refractory MCL. The standard lymphodepletion regimen for brexu-cel is fludarabine (30 mg/m2) and cyclophosphamide (500 mg/m2). Fludarabine was dose-reduced by 50% to 15 mg/m2 with cyclophosphamide maintained at full dose (500 mg/m2). Chemotherapy was scheduled on day −5, day −4, and day −3 in the evening approximately 24 hours apart, with dialysis performed on day −5 and day −3, approximately 12 hours following chemotherapy. In this case we chose to give mesna, as well as to dose reduce cyclophosphamide on day −3 to compensate for the lack of dialysis following this chemotherapy administration. Hunter et al. recently published their experience with CAR T-cell therapy in two ESRD patients with R/R DLBCL.24 Their lymphodepletion strategy differed from ours. In their cases, it was elected to dose-reduce cyclophosphamide 40% to 300 mg/m2 in their axi-cel patient while maintaining the standard cyclophosphamide dose (300 mg/m2) in their liso-cel patient. Additionally, we did not perform dialysis on day −4 in either of our patients, while they performed dialysis 12 hours after each dose that was given. This institutional variability highlights how little is known regarding the optimal dose of fludarabine and cyclophosphamide and the effect of renal dysfunction on these levels. Similarly, it is unknown whether dialysis-dependent patients would benefit from more frequent hemodialysis while experiencing CAR T-cell toxicities such as CRS and ICANS.
CRS is a systemic inflammatory response associated with cellular therapy characterized by increased levels of inflammatory cytokines and activation of effector immune cells.25 This in turn can lead to endothelial dysfunction with vascular leakage and disseminated intravascular coagulopathy that can be seen similarly in patients with overwhelming sepsis.26–28 This distributive phenomenon places the kidneys at risk for hypoperfusion with resultant acute tubular necrosis. Therefore, it is not surprising that CRS was commonly seen in our group of patients with AKIs. Moreover, patients with CRS may have multifactorial risks of AKI due to nephrotoxic treatments, infections, and other factors that can impact hemodynamic status. The association that we observed between AKI and shorter PFS and OS likely relates to AKI being a marker for patients who have high levels of inflammation, poor tolerance of fluid shifts, multiorgan illness, and lymphoma at higher risk of relapse after CAR T cell therapy.
Limitations of this study include its retrospective nature, lack of fludarabine pharmacokinetic data, and incomplete sample representation of cytokine levels. In summary, our study shows similar renal and survival outcomes for R/R DLBCL patients receiving SOC CAR T-cell therapy between patients with or without RI at start of lymphodepletion. Patients with AKIs had decreased PFS and OS as compared to those who did not experience an AKI. CRS is commonly associated with CAR T-cell patients who experience AKIs and occurs with or without baseline RI. Fludarabine dose-reduction status did not have an adverse impact on clinical outcomes. CD19 directed CAR T-cell therapy is feasible in patients with RI including dialysis dependent cases with careful planning of lymphodepletion. However, optimal lymphodepletion strategies still need to be determined regardless of renal function.
Supplementary Material
Acknowledgments
RF reports research function from Kite/Gilead and Novartis. JCC reports consultancy/advisory/honoraria for Kite/Gilead, Novartis, Karyopharm, MorphoSys, BeiGene, Abbvie, ADC Therapeutics, BMS, Epizyme, Genentech, and Bayer, and research funding from AstraZeneca, Merck, and Adaptive. BS reports consultancy/advisory/honoraria for Pfizer, Amgen, BMS, Precision Biosciences, Century Therapeutics, Adaptive Biotech, Biegene, and AstraZeneca, and research funding from Jazz Pharmaceuticals, Kite/Gilead, and Servier. TN reports research funding from Novartis and Karyopharm. AL reports consultancy/advisory for Humanigen, Kadmon, and Sanofi. CF reports consultancy/advisory/honoraria for BMS, Seattle Genetics, Teva, Janssen, Amgen, Celgene, Incyte, Sanofi, and Abbvie, and research funding from Roche Pharmaceuticals and Teva. MLD reports consultancy/advisory/honoraria for Kite/Gilead, Novartis, Atara, Precision Biosciences, Celyad, Bellicum, GSK, Adaptive Biotech, and Anixa Biosciences, and research funding from Kite/Gilead, Novartis, and Atara. FLL reports consultancy/advisory for Allogene, Amgen, Bluebird Bio, BMS/Celgene, Calibr, Cellular Biomedicine Group, Cowen, EcoR1, Emerging Therapy Solutions, GammaDelta Therapeutics, Gerson Lehrman Groupt, Iovance, Kite Pharma, Janssen, Legend Biotech, Novartis, Sana, Takeda, Wugen, and Umoja, and research funding from Kite/Gilead, Allogene, Novartis, BlueBird Bio, BMS, NCI, Leukemia and Lymphoma Society, and the Moffitt Cancer Center support grant (P30-CA076292) and education or editorial activity for Aptitude Health, ASH, BioPharma Communications CARE Education, Clinical Care Options Oncology, Imedex, Society for Immunotherapy of Cancer. MDJ reports consultancy/advisory for Kite/Gilead, Novartis, BMS, and MyeloidTx, and research funding from Incyte and Kite/Gilead.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ACW, APP, BA, KP, GJ, ED, CAB, KR, SC, MHV, SG, FK, HE, RM, and CB have no conflicts of interest to declare.
References
- 1.Neelapu SS, et al. , Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med, 2017. 377(26): p. 2531–2544.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster SJ, et al. , Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med, 2019. 380(1): p. 45–56. [DOI] [PubMed] [Google Scholar]
- 3.Abramson JS, et al. , Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet, 2020. 396(10254): p. 839–852. [DOI] [PubMed] [Google Scholar]
- 4.Locke FL, et al. , Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med, 2022. 386(7): p. 640–654. [DOI] [PubMed] [Google Scholar]
- 5.Kamdar M, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet, 2022. 399(10343): p. 2294–2308. [DOI] [PubMed] [Google Scholar]
- 6.Sehgal A et al. Lisocabtagene maraleucel as second-line therapy in adults with relapsed or refractory large B-cell lymphoma who were not intended for haematopoietic stem cell transplantation (PILOT): an open-label, phase 2 study. Lancet Oncol, 2022. 23(8): p.1066–1077. [DOI] [PubMed] [Google Scholar]
- 7.Launay-Vacher V, et al. , Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer, 2007. 110(6): p. 1376–84. [DOI] [PubMed] [Google Scholar]
- 8.Kittai AS, et al. Comorbidities predict inferior survival in patients receiving chimeric antigen receptor T cell therapy for diffuse large B cell lymphoma: a multicenter Aanalysis. Transplant Cell Ther, 2021. 27(1): p. 46–52. [DOI] [PubMed] [Google Scholar]
- 9.Sorror ML, et al. , Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood, 2005. 106(8): p. 2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lichtman SM, et al. , The pharmacokinetics and pharmacodynamics of fludarabine phosphate in patients with renal impairment: a prospective dose adjustment study. Cancer Invest, 2002. 20(7–8): p. 904–13. [DOI] [PubMed] [Google Scholar]
- 11.Langenhorst JB, et al. , Fludarabine exposure in the conditioning prior to allogeneic hematopoietic cell transplantation predicts outcomes. Blood Adv, 2019. 3(14): p. 2179–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabrizio VA, et al. Optimal fludarabine lymphodepletion is associated with improved outcomes after CAR T-cell therapy. Blood Adv, 2022. 6(7): p. 1961–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chawla LS et al. Acute Disease Quality Initiative Workgroup 16. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol, 2017. 13(4): p. 241–257. [DOI] [PubMed] [Google Scholar]
- 14.Faramand R, et al. Tumor microenvironment composition and severe cytokine release syndrome (CRS) influence toxicity in patients with large B-cell lymphoma treated with axicabtagene ciloleucel. Clin Cancer Res, 2020. 26(18): p. 4823–4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain MD, et al. Tumor interferon signaling and suppressive myeloid cells are associated with CAR T-cell failure in large B-cell lymphoma. Blood, 2021.137(19): p. 2621–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashmi H, et al. Haemophagocytic lymphohistiocytosis has variable time to onset following CD19 chimeric antigen receptor T cell therapy. Br J Haematol, 2019. 187(2): e35–e38. [DOI] [PubMed] [Google Scholar]
- 17.Gutgarts V, et al. Acute kidney injury after CAR-T cell therapy: low incidence and rapid recovery. Biol Blood Marrow Transplant 2020, 26(6): p. 1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta S, et al. Acute kidney injury and electrolyte abnormalities after chimeric antigen receptor T-cell (CAR-T) therapy for diffuse large B-cell lymphoma. Am J Kidney Dis 2020, 76(1): p. 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dekker L, et al. Fludarabine exposure predicts outcome after CD19 CAR T-cell therapy in children and young adults with acute leukemia. Blood Adv, 2022. 6(7):1969–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haubitz M, et al. , Cyclophosphamide pharmacokinetics and dose requirements in patients with renal insufficiency. Kidney Int, 2002. 61(4): p. 1495–501. [DOI] [PubMed] [Google Scholar]
- 21.De Jonge ME, et al. , Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet, 2005. 44(11): p. 1135–64. [DOI] [PubMed] [Google Scholar]
- 22.van Besien K, et al. , Allogeneic stem cell transplant in renal failure: engraftment and prolonged [DOI] [PubMed]
- 23.Dekker L, et al. Fludarabine exposure predicts outcome after CD19 CAR T-cell therapy in children and young adults with acute leukemia. Blood Adv, 2022. 6(7):1969–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter BD, et al. Successful administration of chimeric antigen receptor (CAR) T-cell therapy in patients requiring hemodialysis. Exp Hematol Oncol, 2022.11(1): p10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DW, et al. , ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant, 2019. 25(4): p. 625–638. [DOI] [PubMed] [Google Scholar]
- 26.Hong F, et al. , Predictive role of endothelial cell activation in cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukaemia. J Cell Mol Med, 2021. 25(24): p. 11063–11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzgerald JC, et al. , Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med, 2017. 45(2): p. e124–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, et al. , Coagulation disorders after chimeric antigen receptor T cell therapy: analysis of 100 patients with relapsed and refractory hematologic malignancies. Biol Blood Marrow Transplant, 2020. 26(5): p. 865–875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


