Abstract
Cytomegalovirus (CMV) reactivation is frequent after autologous stem cell transplantation (ASCT), but generalized CMV lymphadenitis is rare. We report a CMV lymphadenitis after an ASCT mimicking a relapse of a diffuse large B‐cell lymphoma. After histopathological documentation, CMV lymphadenitis should be treated to avoid systemic progression.
Keywords: autologous stem cell transplantation, cytomegalovirus, lymphadenitis, lymphoma
Short abstract
CMV lymphadenitis is a rare complication following ASCT, which may mimic a relapse of lymphoma. Blood CMV viremia can be negative. Following histopathological documentation, CMV lymphadenitis should be treated to avoid systemic progression.
1. INTRODUCTION
Cytomegalovirus (CMV) reactivation is a regular event in hematologic malignancies, especially during allogenic stem cell transplantation. During autologous stem cell transplantation (ASCT), CMV reactivation has been reported to occur in up to 41% of seropositive patients. 1 Compromised T‐cell immunity persists for up to 1 year after ASCT so that infection or viral recurrences are frequent during this period. Most of the time CMV reactivation is asymptomatic, but systemic disease can occur with colitis, pneumonia, and keratitis. In the particular case of lymphadenitis, the infection is limited to lymph nodes and can mimic a relapsed neoplasia. 2 We report a rare case of CMV lymphadenitis mimicking lymphoma relapse, which had a rapid favorable outcome only after antiviral treatment.
2. CLINICAL CASE
A 41‐year‐old woman with no noticeable personal or familial past history was diagnosed with an advanced‐stage diffuse large B‐cell lymphoma (DLBCL). At diagnosis, she had palpable axillary nodes and nocturnal fever. Initial positron emission tomography and computed tomography (PET‐CT) showed hypermetabolic cervico‐axillary lymph nodes with splenic and bone lesions. First‐line treatment consisted of 8 courses of R‐CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) resulting in a complete metabolic response. Final disease reevaluation confirmed complete metabolic response. Three years later, she experienced a biopsy‐proven relapse with palpable axillary and cervical nodes. PET‐CT found supra‐ and infra‐diaphragmatic lymph nodes (maximal standardized uptake values [SUVmax] 37.8) with hepatic, splenic, and bone involvement. She received salvage therapy consisting of 4 courses of R‐DHAP regimen (rituximab, dexamethasone, and high‐dose cytarabine, cisplatin) with complete metabolic response after 2 courses. She then was consolidated with high‐dose chemotherapy (carmustine, etoposide, cytarabine, and melphalan) followed by autologous stem cell rescue. During hospitalization, she experienced a febrile neutropenia without any bacterial or viral documentation of favorable outcome with a probabilistic broad‐spectrum antibiotic therapy for 5 days. PET‐CT reevaluation after 2 months confirmed complete metabolic response. Seven months later (i.e., 9 months following ASCT), supra‐ and infra‐diaphragmatic lymph nodes were found on PET‐CT (SUV max 9.9). The patient had no related symptoms. Lymphocyte count was 2.2 G/L including 68%, 26%, and 42% CD3+, CD4+, and CD8+ T‐cells, respectively, 22% CD19+ B‐cells, and 8.5% CD16 + CD56+ natural killer cells. Serum LDH level was normal. Serum CMV IgG were positive, consistent with a past infection.
A surgical biopsy of a sub‐clavicular lymph node showed unspecific follicular hyperplasia. Viral eosinophilic nuclear inclusions were detected next to monocytoid B‐cell hyperplasia in interfollicular area (Figure 1). Immunohistochemistry using an anti‐cytomegalovirus antibody revealed CMV‐infected cells. Blood CMV polymerase chain reaction (PCR) was negative. The patient did not develop colitis or retinitis. These results were consistent with a CMV lymphadenitis. The patient was first watched, but a repeat PET‐CT scan after 3 months revealed progressive disease with increased SUV (SUmax 14.3) and new hypermetabolic lymph nodes. Blood CMV PCR was again negative. Based on these new findings, the patient received ganciclovir 5 mg/kg twice a day for 15 days. No treatment‐related adverse event occurred. Reevaluation by PET‐CT 1 month later revealed a complete response in the pulmonary and pectoral lymph nodes and a near‐complete response in the axillary lymph nodes (SUVmax 3.3 versus 7.6).
FIGURE 1.
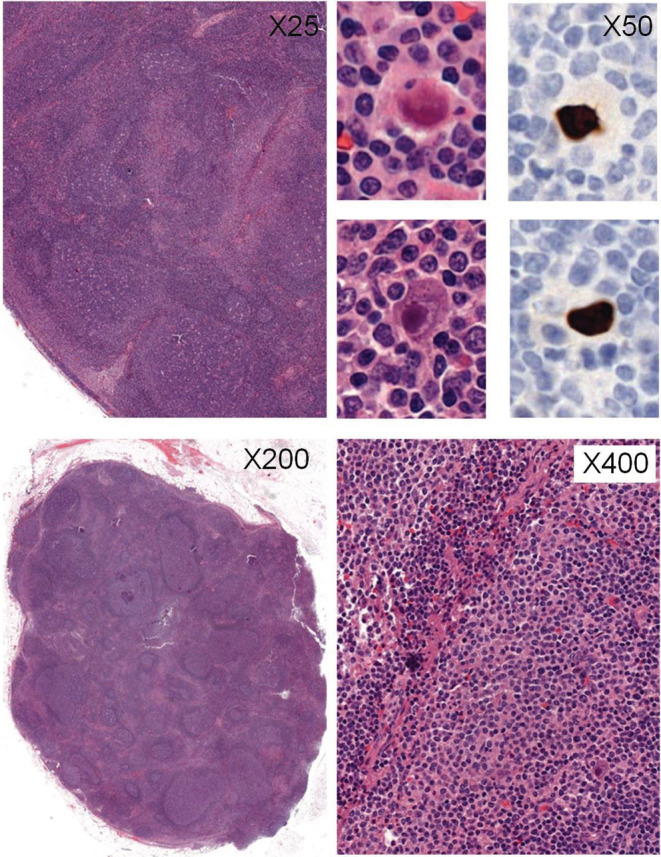
Lymph node biopsy. Histopathological analysis showed a reactive lymph node with follicular reactional hyperplasia (upper left ×25 and right ×50), monocytoid B‐cell hyperplasia (upper right ×50 and bottom left ×200) and sparse enlarged cells with viral eosinophilic inclusions (bottom left ×200 and right ×400), immunohistochemically stained with CMV antibody (bottom right ×400).
3. DISCUSSION
We present here a case of a diffuse CMV lymphadenitis that occurred 7 months after ASCT in a patient with a DLBCL. The patient had no related symptoms and the PET‐CT mimicked a DLBCL relapse, confirming the need to systematically perform a biopsy to ascertain a relapse. As T‐cell repertoire recovery following ASCT typically occurs more than one year later, 3 infectious lymphadenitis is thereby a diagnostic that needs to be considered. Other infectious agents including Epstein–Barr virus (EBV), toxoplasmosis and mycobacteria should also be ruled out. Viral blood PCR can help in the diagnosis, but as illustrated in our case, a negative blood PCR does not exclude the diagnosis of viral adenitis and only histological analysis can rule out or confirm the diagnosis. 2 There are only few reports of CMV lymphadenitis. 2 , 4 Whether a systematic anti‐CMV agent must be introduced in this setting remains a matter of controversy. Recommendations only focus on the treatment of active forms such as pneumonia. 5 Lymphadenitis, a rare condition which outcome is still unclear, is not mentioned. While cases of spontaneous resolution were reported, 2 our patient experienced a progression of lymphadenitis after a 3‐month observation that resolved rapidly and durably following antiviral treatment initiation. In addition, the monitoring of blood CMV PCR and the pre‐emptive treatment of asymptomatic CMV reactivations, which are standard after allogeneic transplantation, are not consensual after ASCT. 6
In conclusion, CMV lymphadenitis is a rare complication that should be considered following ASCT. As clinical presentation can mimic a lymphoma relapse, histopathological confirmation is needed. Antiviral treatment may be required to prevent a systemic progression of the disease.
AUTHOR CONTRIBUTIONS
MD and LR drafted the article. SC contributed to provision of figure. LM, SC, and PC contributed to proofreading of the manuscript. PC contributed to project supervision.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
None.
Delaye M, Ricard L, Maisonobe L, Chartier S, Coppo P. Cytomegalovirus lymphadenitis mimicking a relapsed diffuse large B‐cell lymphoma. Clin Case Rep. 2022;10:e06611. doi: 10.1002/ccr3.6611
DATA AVAILABILITY STATEMENT
Not Applicable.
REFERENCES
- 1. Marchesi F, Pimpinelli F, Ensoli F, Mengarelli A. Cytomegalovirus infection in hematologic malignancy settings other than the allogeneic transplant. Hematol Oncol. 2018;36(2):381‐391. doi: 10.1002/hon.2453 [DOI] [PubMed] [Google Scholar]
- 2. Kang KW, Lee JH, Choi JS, et al. Spontaneous resolution of post‐transplant localized cytomegalovirus lymphadenitis mimicking tumor recurrence. Transpl Infect Dis. 2014;16(4):676‐680. doi: 10.1111/tid.12254 [DOI] [PubMed] [Google Scholar]
- 3. Steingrimsdottir H, Gruber A, Björkholm M, Svensson A, Hansson M. Immune reconstitution after autologous hematopoietic stem cell transplantation in relation to underlying disease, type of high‐dose therapy and infectious complications. Haematologica. 2000;85(8):832‐838. [PubMed] [Google Scholar]
- 4. Yu SC, Ko KY, Teng SC, et al. A clinicopathological study of cytomegalovirus lymphadenitis and tonsillitis and their association with Epstein‐Barr virus. Infect Dis Ther. 2021;10:2661‐2675. doi: 10.1007/s40121-021-00528-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ljungman P, de la Camara R, Cordonnier C, et al. Management of CMV, HHV‐6, HHV‐7 and Kaposi‐sarcoma herpesvirus (HHV‐8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant. 2008;42(4):227‐240. doi: 10.1038/bmt.2008.162 [DOI] [PubMed] [Google Scholar]
- 6. Kaya AH, Tekgunduz E, Akpinar S, et al. Is cytomegalovirus surveillance necessary for patients with low reactivation risk in an autologous hematopoietic cell transplantation setting? Transplant Proc. 2017;49(8):1911‐1915. doi: 10.1016/j.transproceed.2017.05.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.


