Abstract
Studies have demonstrated that addiction leads to blunted responses of cortisol and sympathetic nervous system (SNS) to acute stressors; however, limited studies have examined the neuroendocrine and SNS stress responses in Internet addiction (IA). To examine acute stress responses in IA, the current study recruited a total of 76 Japanese university students and staff members (51 females and 25 males, mean age = 22.4 years, SD = 4.7), and measured the salivary cortisol, salivary alpha-amylase (sAA), and blood pressure (BP) responses to an acute stressor under stress or a nonstress placebo conditions in IA and non-IA groups. The results revealed that patients with IA showed a blunted cortisol response to a stressor. In contrast, no differences were found in the sAA and BP responses between the IA and non-IA groups. These results suggest that IA may be characterized by blunted cortisol responses in acute stress settings.
Keywords: Internet addiction, Cortisol, Alpha-amylase, Blood pressure, Acute stress
Internet addiction; Cortisol; Alpha-amylase; Blood pressure; Acute stress.
1. Introduction
With the widespread use of Internet, Internet addiction (IA) has become a global issue. IA is a non-substance behavioral addiction defined as excessive or poorly controlled preoccupations, urges, or behaviors related to Internet use, accompanying distress and functional impairment in several life domains (Weinstein et al., 2014). The term "IA" refers to generalized addictive use of the Internet, regardless of specific online activities. Addictive use of online content such as video games and social networking services (SNS) is categorized as an IA subtype, referred to as Internet gaming disorder (IGD) and social networking addiction.
Prior studies have demonstrated a close association between addiction and stress (Sinha, 2008). Individuals with substance and behavioral addictions have been affirmed to have higher levels of psychological stress responses (i.e., perceived negative feeling) (Sinha and Jastreboff, 2013). IA is also associated with higher psychological stress (Kuss et al., 2021; Tsumura et al., 2018). A laboratory stress task evoked higher levels of acute psychological stress in individuals addicted to alcohol (Bernardy et al., 2003; Sinha et al., 2009), opioids (Back et al., 2015), heroin (Gerra et al., 2014), and gambling (Kaess et al., 2017), whereas some studies reported normal levels of psychological stress responses to acute stressors in addiction (Back et al., 2008; Ginty et al., 2012; Panknin et al., 2002). Stress responses and exposure to stressors are also related to addiction (Turner and Lloyd, 2003). For instance, previous studies have revealed that individuals who experience trauma, such as childhood abuse, natural disaster, and serving as a soldier, are more likely to develop an addiction in the future (al’Absi et al., 2021; Goeders, 2003; Levin et al., 2021). Furthermore, stress-related psychiatric disorders such as posttraumatic stress disorder (PTSD) are more commonly seen to co-occur with addiction (Briand and Blendy, 2010; Bruijnzeel, 2012; Donovan et al., 2001; Saxon et al., 2001).
Altered neuroendocrine and autonomic nervous system (ANS) responses to acute stressors are linked to addiction. Research has been conducted in humans that focused on the two main stress systems of organisms, namely, the hypothalamic–pituitary–adrenal (HPA) axis and ANS. Although some studies have reported exaggerated (Starcke et al., 2013) and normal (Back et al., 2015) cortisol and sympathetic nervous system (SNS) responses, substance addiction has been linked to blunted cortisol, the final product of the HPA axis, and SNS responses to acute stressors (Chen et al., 2020). Individuals with blunted cortisol responses are reportedly addicted to alcohol (Ehrenreich et al., 1997; Sinha et al., 2009), heroin (Gerra et al., 2014), and nicotine (Back et al., 2008; Ginty et al., 2014). In line with substance addiction, studies have validated that behavioral addiction is also associated with blunted cortisol and SNS responses to acute stressors; blunted cortisol and SNS stress responses have been linked to gambling disorder (Paris et al., 2010), binge eating (Ginty et al., 2014), and excessive exercising (Heaney et al., 2011).
Limited studies have reported the relationship between IA and neuroendocrine and ANS stress responses. Bibbey et al. (2015) verified that university students with IA showed normal salivary cortisol responses to an acute psychosocial stressor. However, the study measured salivary cortisol only once after completing the stress task, and it may therefore have failed to capture the cortisol response at its peak. Kaess et al. (2017) reported that youth with IGD exhibited blunted cortisol responses to an acute psychosocial stressor. IGD is an addiction specific to online gaming; therefore, a cortisol stress response in IA, which is characterized by the addictive use of the Internet in general with no specific online content, has not been reported. Furthermore, prior studies on neuroendocrine and ANS stress responses to behavioral addiction did not include a nonstress placebo condition; thus, the potential confounding variables could decrease the internal and statistical conclusion validity of the results.
The current study examined the HPA axis and SNS responses to an acute psychological stressor in IA. This study analyzed the levels of salivary cortisol and alpha-amylase (sAA) and blood pressure (BP) to evaluate the HPA axis and the SNS activities, respectively. Adults with and without IA were placed under stress or nonstress placebo conditions to exclude potential confounding variables. Cumulative evidence (e.g., Carroll et al., 2017; Chen et al., 2020) suggests that substance and behavioral addictions are related to blunted cortisol and SNS stress responses; therefore, it was hypothesized that individuals with IA will present with blunted cortisol, sAA, and BP responses.
2. Methods
2.1. Participants
Participants were recruited from among the students or staff of the Shimane University through advertisement at the university campus. A total of 76 Japanese university students and staff members (51 females and 25 males) participated in the study. The mean age was 22.4 years (standard deviation [SD] = 4.7), ranging from 18 to 39 years old. The exclusion criteria were as follows: aged <18 or >39 years; having acute or chronic somatic or psychiatric disorders; taking medication that are associated with cortisol and SNS responses; and female with irregular menstrual cycles or taking oral contraceptive. Participants were compensated for their participation. The participants were stratified by sex and randomly allocated to the stress condition, with acute stress tasks (25 females and 13 males) or the nonstress placebo condition, with placebo tasks (26 females and 12 males). Furthermore, according to the Internet addiction test (IAT; Young, 1998; Osada, 2013) as described below, the participants were post-hoc allocated to the IA group (IAT score ≥50) or the non-IA group (IAT score <50) (Tateno et al., 2018). The study was approved by the Medical Research Ethics Committee, Shimane University Faculty of Medicine (No. 20161130-2, approved on December 14, 2016). All the participants received detailed information about the study and provided written informed consent prior to their participation.
2.2. Measurements
2.2.1. Participant background
The participants provided information regarding sex, age, height, body weight, the number of days after menstruation (for females), and the amount and frequency of alcohol consumption and smoking.
2.2.2. IA
The severity of IA was assessed using the Japanese version of the Internet addiction test (IAT; Young, 1998; Osada, 2013), a self-reported questionnaire comprising items that evaluate the impact of Internet use on daily life, social functioning, and feelings. The reliability and validity of the questionnaire was reported to be adequate (Osada, 2013; Widyanto and McMurran, 2004). Each item was rated using a 5-point Likert scale, and the total scores on the IAT ranged from 20 to 100. In this sample, the Cronbach's α coefficient was 0.92.
2.2.3. Depressive symptoms
The Japanese version of the Center for Epidemiologic Studies—Depression (CES-D scale; Radloff, 1977; Shima et al., 1985) is a self-reported questionnaire composed of 20 items evaluating depressive symptoms experienced during the past week. The CES-D comprised items including, "I felt depressed," and" I felt sad." Each item on the scale is rated on a 4-point Likert scale ranging from 0 (less than 1 day) to 3 (5–7 days). The total score on the scale ranges from 0 to 60. The scale has confirmed validity and reliability (Radloff, 1977; Shima et al., 1985). In this sample, the Cronbach's α coefficient was 0.88.
2.2.4. Trait anxiety
Trait anxiety was measured using the Japanese version of the Spielberger State-Trait Anxiety Inventory's—trait form (STAI-T; Spielberger et al., 1997; Shimizu and Imae, 1981). The STAI-T is a 20-item self-reported questionnaire comprising statements such as, "I feel pleasant," and" I feel secure." The respondents indicate how they experienced these conditions generally. Responses are made using a 4-point Likert scale ranging from 1 (almost never) to 4 (almost always)—the total scores on the scale range from 20 to 80. The scale has demonstrated validity and reliability (Spielberger et al., 1997; Shimizu and Imae, 1981). In this sample, the Cronbach's α coefficient was 0.90.
2.2.5. Perceived stress
The Japanese version of the Perceived Stress Scale (PSS; Cohen et al., 1983; Iwahashi et al., 2002) is a self-reported questionnaire for assessing perceived levels of stress. The scale comprises 14 items concerning feelings and thoughts about unpredictability, uncontrollability, and being overloaded during the last month. The items include, "How often have you been upset because of something that happened unexpectedly?" and "How often have you been able to control irritations in your life?" Each item is rated on a 5-point Likert scale ranging from 0 (never) to 4 (very often)—the total scores on this scale range from 0 to 56. The scale has demonstrated validity and reliability (Cohen et al., 1983; Iwahashi et al., 2002). In this sample, the Cronbach's α coefficient was 0.86.
2.2.6. Affective response
Affective responses were assessed using the Japanese version of the positive and negative affect schedule (PANAS; Watson et al., 1988; Sato and Yasuda 2001), a self-reported scale comprising two subscales to measure the positive (PANAS-P) and negative (PANAS-N) affects. The Japanese version of the PANAS comprised 16 items; 8 positive and negative mood terms each. The current affect was rated on a 6-point scale, and the sum of the ratings generated the positive and negative affect scores that each ranged from 8 to 48. The questionnaire was confirmed to be reliable and valid (Watson et al., 1988; Sato and Yasuda 2001). In this sample, the Cronbach's α coefficients of PANAS-P and PANAS-N ranged from 0.90 to 0.92 and 0.86 to 0.92, respectively.
2.2.7. HPA axis and SNS responses
For the assessment of salivary cortisol and sAA levels, saliva samples were collected through passive drooling during the time period from 13:00 to 17:00 h. Participants were asked to refrain from alcohol, caffeine, and drug consumption and smoking a day before the experiment. They were also required to abstain from eating, brushing, and excessive physical activity 1 h before the experiment. The saliva samples were frozen below −80 °C until assay. The salivary cortisol and sAA levels were measured via enzyme-linked immunoassay and kinetic enzyme assay using commercial kits from Salimetrics (State College, PA, USA) according to the manufacturer's instruction. The detection limits are 0.012 μg/dl and 2 U/ml for cortisol and sAA, respectively. The measurement was conducted in duplicates. The inter- and intra-assay coefficients for the assay were <10%. Samples from the same participants were analyzed in using the same assay. To measure BP, the systolic BP (SBP) and diastolic BP (DBP) were recorded using a BP meter from A&D Medical (Tokyo, Japan).
2.3. Acute psychosocial stress task
We used the paced auditory serial addition test modified for stress induction inducing stress because it can successfully elicit affective, neuroendocrine and ANS stress responses (Ginty et al., 2012; Heaney et al., 2011). In accordance with prior studies (Ginty et al., 2012; Heaney et al., 2011), a series of single numbers was serially presented on a laptop. Participants were instructed to speak aloud the sum of the current and previously displayed numbers. The task comprised five blocks and lasted for approximately 15 min. The intervals between the numbers were 3.5 s for the first block, and reduced by 0.5 s for each successive block. A brief burst of approximately 80 db white noise was presented each time a mistake or an omission was made. In addition, the first scores of 100 points were reduced by one point for every wrong answer or omission, and the final point was used as their task performance score. Moreover, the participants were made to compete with their peers on the task performance scores. The current scores were presented to the participants on another laptop PC display. Participants were also videotaped during the task and informed that body language experts had assessed their nonverbal behavior. A mirror was placed approximately 0.5 m in front of them to allow them to see their own reflection from the shoulder up.
2.4. Nonstress placebo task
A nonstress placebo task was designed to be as similar as possible to the stress task but without its effective components (i.e., high cognitive load, error feedback with aversive noise, and social evaluation). The participants were presented with the same number sequence at the same intervals on the same laptop as the stress task. However, in contrast to the stress task and to remove its effective components, the participants were asked to speak aloud each number being displayed on the monitor rather than the sum of the number and its previous number. No aversive noise was presented after participants’ response, and the participants were not placed in the context of peer competition and behavioral assessment. There were no video cameras and mirrors set up for this group.
2.5. Procedure
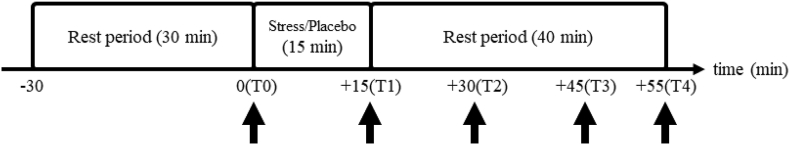
Figure 1 presents the overview of the procedure. Initially, participants rinsed their mouth with water and were asked to remain seated in a quiet room for 30 min during which they completed the self-reported questionnaires. Immediately after the rest period (T0), their BP was measured, saliva samples were taken, and they were asked to complete the PANAS. Thereafter, they were administered the stress task or the nonstress placebo task for approximately 15 min. This was followed by a 40-min rest period during which their BP was measured again, saliva samples were taken, and they were asked to complete the PANAS at +0 (T1), +15 (T2), +30 (T3), and +40 (T4) after the offset of the task. Finally, they were debriefed about the experiment.
Figure 1.
Overview of the experimental procedure. Arrows indicate timing of blood pressure measurement, saliva sampling, and completion of the Japanese version of the positive and negative affect schedule (PANAS).
2.6. Statistical analyses
The participants' background and psychological variables were compared between the groups using unpaired t-tests with Welch's correction, Mann–Whitney's U tests, or a Fisher's exact test. The cortisol and sAA values were log-transformed to reduce the skewness. To test the group differences in cortisol, sAA, and BP values and the PANAS scores, the three-way mixed-design analyses of variance (ANOVA) with group (IA and non-IA) and stress (stress and placebo) as between factors and time (T0, T1, T2, T3, and T4) as within factors were conducted. With the violation of the assumption of sphericity, Greenhouse–Geisser procedures were applied to correct the results. In case of a significant interaction, the analyses were followed by post-hoc pairwise comparisons using the Holm method. Partial correlation analyses were used to quantify associations between IAT scores and cortisol levels at T1, T2, T3, and T4 controlling for cortisol levels at T0 in the stress condition. We also reported partial eta squared (ηp2) as the effect size, and all the probability values were two-tailed and at a 5% level of significance. Due to the exploratory nature of this study, no adjustments for multiple testing were applied. HAD (version 17.202; Shimizu, 2016) was used for the analyses.
3. Results
3.1. Group characteristics
After the classification on the basis of the IAT scores, 10 participants in the IA group and 28 participants in the non-IA group were placed on stress condition and 10 participants in the IA group and 28 participants in the non-IA group were placed on nonstress placebo condition. Table 1 presents the means and SDs of participant background and psychological variables for stress and placebo conditions in the IA and non-IA groups. The IA group had higher IAT, CES-D, STAI-T, and PSS scores. There were no differences in age, sex, BMI, number of days after menstruation, and the amount and frequency of alcohol drinking and smoking between the groups.
Table 1.
Means and standard deviations of the participant background variables for the Internet addiction and non-Internet addiction groups.
| IA |
Non-IA |
Group differences IA (total) versus non-IA (total) | |||||
|---|---|---|---|---|---|---|---|
| Total (n = 20) | Stress (n = 10) | Placebo (n = 10) | Total (n = 56) | Stress (n = 28) | Placebo (n = 28) | ||
| Age (years), M(SD) | 21.2 (4.4) | 19.7 (1.3) | 22.6 (5.9) | 22.9 (4.8) | 23.1 (5.2) | 22.6 (4.6) | t (36.3) = 1.44, p = 0.16 |
| Sex (males/females) | 5/15 | 3/7 | 2/8 | 20/36 | 10/18 | 10/18 | p = 0.42 |
| BMI (kg/m2), M(SD) | 21.3 (3.5) | 21.4 (2.9) | 21.3 (4.2) | 22.1 (2.8) | 22.0 (2.4) | 22.1 (3.2) | t (24.6) = 0.85, p = 0.41 |
| Days after menstruation, M(SD) | 21.3 (12.0) | 23.8 (13.2) | 18.8 (11.3) | 18.2 (11.8) | 17.9 (13.4) | 18.6 (10.3) | t (19.2) = 0.77, p = 0.45 |
| Drinking frequency (days/week) | U (1) = 531.0, p = 0.62 | ||||||
| >4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3–4 | 0 | 0 | 0 | 2 | 1 | 1 | |
| 1–2 | 3 | 2 | 1 | 9 | 6 | 3 | |
| <1 | 17 | 8 | 9 | 45 | 21 | 24 | |
| Drinking quantity per drinking day (units/day) | U (1) = 558.0, p = 0.98 | ||||||
| >6 | 1 | 0 | 1 | 3 | 2 | 1 | |
| 5–6 | 1 | 1 | 0 | 8 | 3 | 5 | |
| 3–4 | 7 | 3 | 4 | 12 | 4 | 8 | |
| <3 | 11 | 6 | 5 | 33 | 19 | 14 | |
| Smoking frequency (days/week) | U (1) = 570.0, p = 0.53 | ||||||
| >4 | 0 | 0 | 0 | 1 | 1 | 0 | |
| 3–4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1–2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| <1 | 20 | 10 | 10 | 55 | 27 | 28 | |
| Cigaretts per day, M(SD) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.1 (0.5) | 0.1 (0.8) | 0.0 (0.0) | t (55.0) = 1.00, p = 0.32 |
| IAT score, M(SD) | 61.5 (10.2) | 64.4 (12.7) | 58.5 (6.1) | 35.2 (7.1) | 33.7 (6.6) | 36.6 (7.4) | t (26.0) = 10.67, p < 0.01 |
| CES-D score, M(SD) | 20.7 (10.3) | 16.5 (8.8) | 24.9 (10.5) | 11.9 (7.4) | 13.0 (8.6) | 10.9 (5.9) | t (26.2) = 3.49, p < 0.01 |
| STAI-T score, M(SD) | 54.6 (11.1) | 53.3 (10.7) | 55.8 (11.9) | 43.3 (9.9) | 44.2 (10.4) | 42.5 (9.5) | t (30.5) = 4.00, p < 0.01 |
| PSS score, M(SD) | 31.4 (9.5) | 27.9 (8.7) | 34.8 (9.5) | 22.3 (8.3) | 23.0 (7.9) | 21.6 (8.8) | t (30.0) = 3.75, p < 0.01 |
IA: Internet addiction, IAT: the Japanese version of the internet addiction test, CES-D: the Japanese version of the Center for Epidemiologic Studies—Depression Scale, STAI-T: the Japanese version of the Spielberger State Trait Anxiety Inventory—Trait Form, PSS: the Japanese version of the Perceived Stress Scale.
3.2. Positive and negative affects
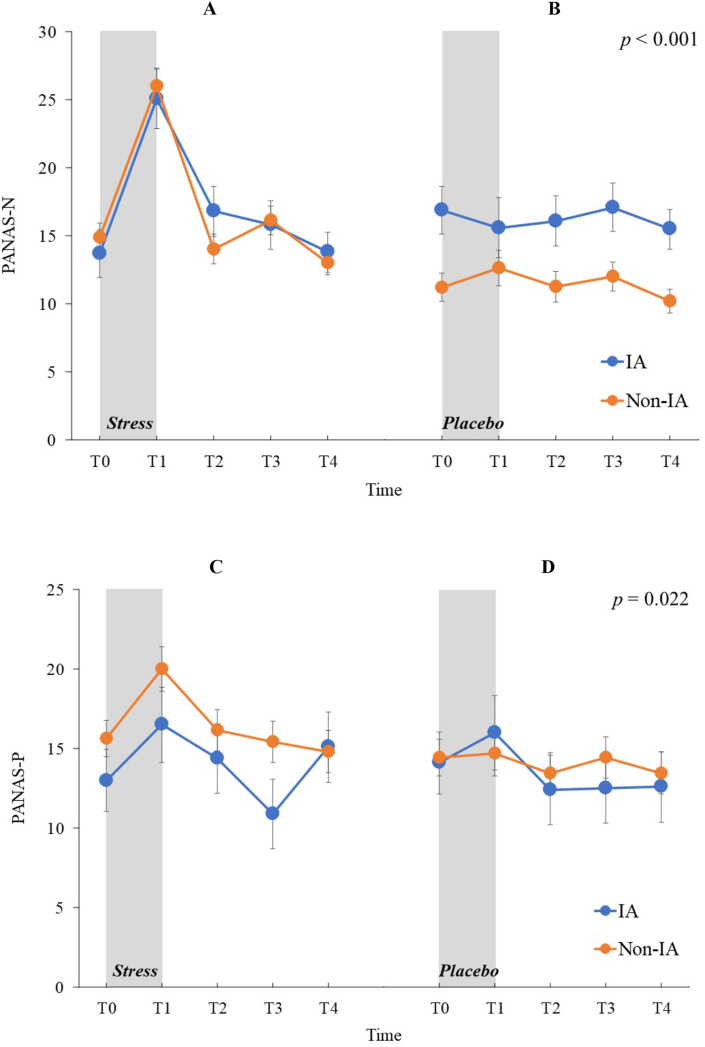
Mixed-design ANOVA for PANAS-N scores revealed that main effects of stress (F (1,72) = 5.83, p = 0.018, ηp2 = 0.08), time (F (4,288) = 33.91, p < 0.001, ηp2 = 0.32) and stress × time interaction were significant (F (4,288) = 27.72, p < 0.001, ηp2 = 0.28; Figure 2) but no other main effects or interactions reached significant (ps > 0.053). In the stress condition, the simple main effect of time was significant (F (4,296) = 82.53, p < 0.001, ηp2 = 0.69) but not in the placebo condition (F (4,296) = 1.78, p = 0.157, ηp2 = 0.04). Post-hoc comparisons indicated that under stress condition, PANAS-N scores at T1 were higher than those at T0 (t (74) = 10.44, p < 0.001), T2 (t (74) = 11.18, p < 0.001), T3 (t (74) = 11.00, p < 0.001), and T4 (t (74) = 12.56, p < 0.001), and the PANAS-N scores at T4 were lower than those at T2 (t (74) = 2.89, p = 0.025) and T3 (t (74) = 5.02, p < 0.001).
Figure 2.
Positive and negative affective schedule score means for the Internet addiction and non-Internet addiction groups under stress and nonstress placebo conditions at five sampling points. (A) Negative affect subscale scores under stress condition. (B) Negative affect subscale scores under nonstress placebo condition. (C) Positive affect subscale scores under stress condition. (D) Negative affect subscale scores under nonstress placebo condition. Error bars indicate SEM. IA: Internet addiction, PANAS-N: negative affect subscale of the Japanese version of the positive and negative affect schedule, PANAS-P: positive affect subscale of the Japanese version of the positive and negative affect schedule. Statistical significance as revealed by three-way mixed-design ANOVA.
Mixed-design ANOVA for PANAS-P scores revealed that a main effect of time (F (4,288) = 12.05, p < 0.001, ηp2 = 0.14) and stress × time interaction were significant (F (4,288) = 3.40, p = 0.022, ηp2 = 0.05; Figure 2) but no other main effects or interactions reached significant (ps > 0.109). In the stress condition, the simple main effect of time was significant (F (4,296) = 15.66, p < 0.001, ηp2 = 0.30) but not in the placebo condition (F (4,296) = 2.58, p = 0.059, ηp2 = 0.06). Post-hoc comparisons indicated that under stress conditions, PANAS-P scores at T1 were higher than those at T0 (t (74) = 5.16, p < 0.001), T2 (t (74) = 4.36, p < 0.001), T3 (t (74) = 6.62, p < 0.001), and T4 (t (74) = 4.40, p < 0.001).
3.3. HPA axis and SNS responses
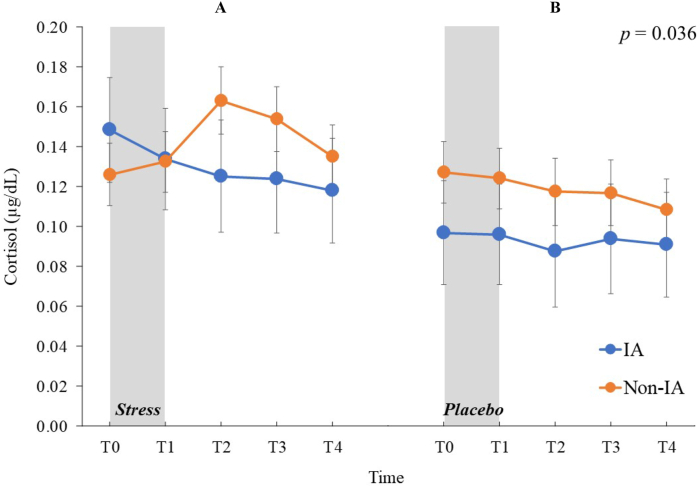
Mixed-design ANOVA for cortisol values revealed that the group × stress × time interaction was significant (F (4,288) = 3.16, p = 0.036, ηp2 = 0.04; Figure 3) but no other main effects or interactions reached significant (ps > 0.104). Simple interaction analyses revealed that the stress × time interaction in the non-IA group was significant (F (4,288) = 6.12, p = 0.001, ηp2 = 0.10) but not in the IA group (F (4,288) = 0.61, p = 0.570, ηp2 = 0.03). In the former, the main effect of time in the stress condition (F (4,288) = 7.49, p < 0.001, ηp2 = 0.22) and of stress at T2 (F (1,360) = 4.15, p = 0.042, ηp2 = 0.07) were significant, but no other main effects or interactions were not (ps > 0.096). Post-hoc comparisons indicated that among the non-IA groups in the stress condition, the cortisol values at T2 were higher than those at T0 (t (72) = 3.69, p = 0.003), T1 (t (72) = 4.12, p = 0.001), and T4 (t (72) = 3.72, p = 0.003), and those at T3 were higher than those at T0 (t (72) = 2.78, p = 0.035), T1 (t (72) = 2.86, p = 0.033), and T4 (t (72) = 3.42, p = 0.007).
Figure 3.
Means of the salivary cortisol levels in μg/dL for the Internet addiction and non-Internet addiction groups under stress and nonstress placebo conditions at five sampling points (untransformed raw data). (A) Salivary cortisol levels under stress condition. (B) Salivary cortisol levels under nonstress placebo condition. Error bars indicate SEM. IA: Internet addiction. Statistical significance as revealed by three-way mixed-design ANOVA.
Additional partial correlation analyses revealed that in the stress condition, IAT scores were negatively correlated with cortisol levels at T2 (r = −0.34, p = 0.035) and T3 (r = −0.33, p = 0.049), controlling for levels at T0. Partial correlation coefficients were not significant between IAT scores and cortisol levels at T1 and T4 (ps > 0.101).
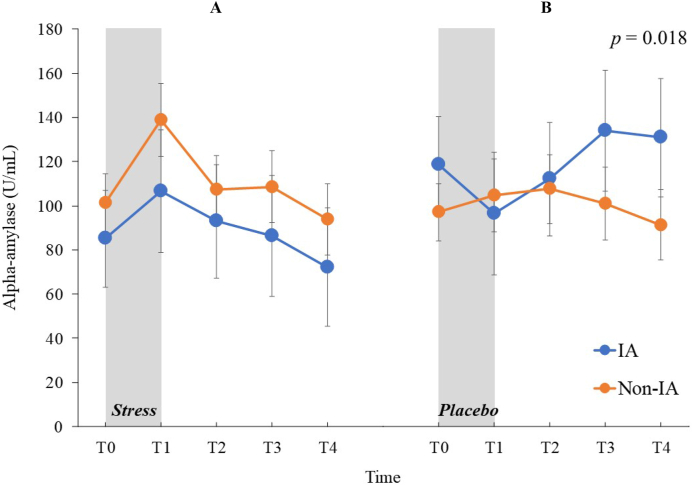
Mixed-design ANOVA for sAA values revealed that the stress × time interaction was significant (F (4,288) = 3.21, p = 0.018, ηp2 = 0.04; Figure 4) but no other main effects or interactions reached significant (ps > 0.089). In the stress condition, the simple main effect of time was significant (F (4,296) = 5.59, p = 0.001, ηp2 = 0.13) but not in the placebo condition (F (4,296) = 1.02, p = 0.390, ηp2 = 0.03). Post-hoc comparisons indicated that in the stress condition, the sAA values at T1 were higher than those at T0 (t (74) = 3.30, p = 0.013) and T4 (t (74) = 4.04, p = 0.001).
Figure 4.
Means of the salivary alpha-amylase levels in U/mL for the Internet addiction and non-Internet addiction groups under stress and nonstress placebo conditions at five sampling points (untransformed raw data). (A) Salivary alpha-amylase levels under stress condition. (B) Salivary alpha-amylase levels under nonstress placebo condition. Error bars indicate SEM. IA: Internet addiction. Statistical significance as revealed by three-way mixed-design ANOVA.
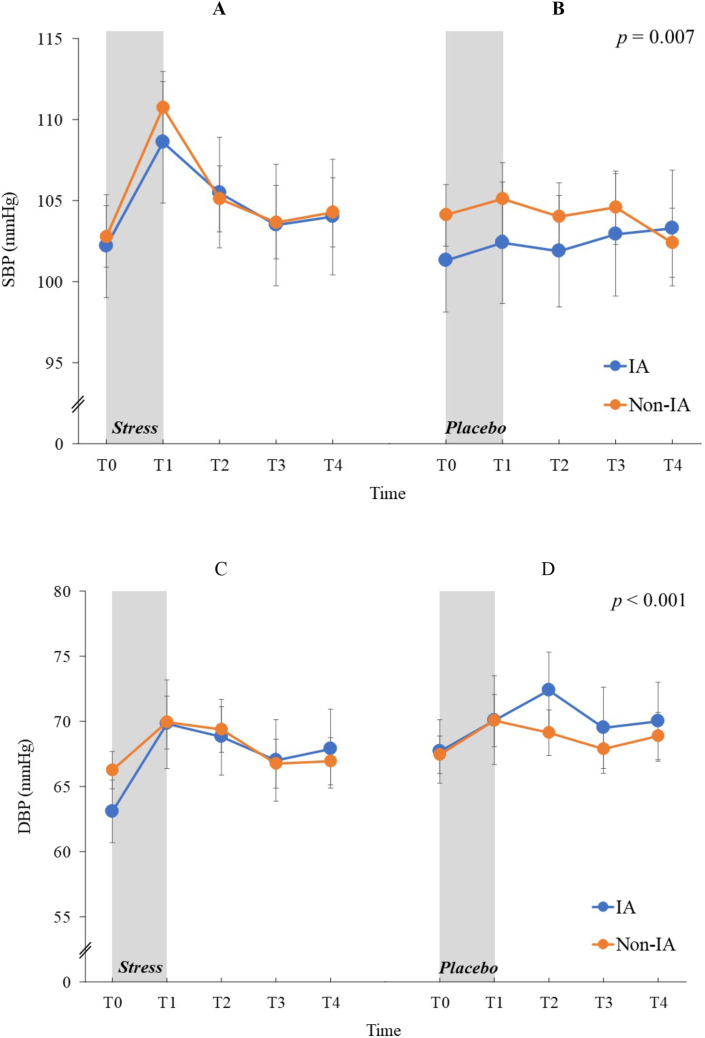
Mixed-design ANOVA for SBP values revealed that the main effect of time (F (4,288) = 5.74, p < 0.001, ηp2 = 0.07) and a stress × time interaction were significant (F (4,288) = 3.78, p = 0.007, ηp2 = 0.05; Figure 5) but no other main effects or interactions reached significant (ps > 0.496). In the stress condition, the simple main effect of time was significant (F (4,296) = 13.59, p < 0.001, ηp2 = 0.27) but not in the placebo condition (F (4,296) = 0.73, p = 0.560, ηp2 = 0.02). Post-hoc comparisons indicated that in the stress condition, the SBP values at T1 were higher than those at T0 (t (74) = 7.38, p < 0.001), T2 (t (74) = 4.08, p = 0.001), T3 (t (74) = 5.26, p < 0.001), and T4 (t (74) = 4.80, p < 0.001) and that the SBP values at T2 were higher than those at T0 (t (74) = 2.99, p = 0.022).
Figure 5.
Means of the systolic and diastolic blood pressure in mmHg for the Internet addiction and non-Internet addiction groups under stress and nonstress placebo conditions at five sampling points. (A) Systolic blood pressure under stress condition. (B) Systolic blood pressure under nonstress placebo condition. (C) Diastolic blood pressure under stress condition. (D) Diastolic blood pressure under nonstress placebo condition. Error bars indicate SEM. IA: Internet addiction, SBP: systolic blood pressure, DBP: diastolic blood pressure. Statistical significance as revealed by three-way mixed-design ANOVA.
Mixed-design ANOVA for DBP values revealed that the main effect of time was significant (F (4,288) = 6.09, p < 0.001, ηp2 = 0.08; Figure 5) but no other main effects or interactions reached significant (ps > 0.429). Post-hoc comparisons indicated that the DBP values at T0 was lower than those at T1 (t (72) = 4.26, p = 0.001), T2 (t (72) = 4.85, p < 0.001), and T4 (t (72) = 3.13, p = 0.020).
4. Discussion
The study examined the HPA axis and SNS stress responses in IA. Individuals with IA have been hypothesized to show blunted HPA axis (i.e., cortisol) and SNS (i.e., sAA and BP) responses. In support of our hypotheses, the results revealed that individuals with IA had blunted cortisol responses. Inconsistent with our hypotheses, no differences were found in the sAA and BP values between the IA and non-IA groups. To the best of our knowledge, this is the first study that demonstrates that IA is linked to blunted cortisol responses to acute stressors. These results suggest that IA may be characterized by blunted cortisol responses in acute stress settings.
The results demonstrated that depression, trait anxiety, and perceived stress are higher in IA, supporting prior studies (e.g., Alpaslan et al., 2016; Prizant-Passal et al., 2016). Additionally, negative affect acutely increased in response to the stressful task, supporting the validity of the stress task used in this study. However, the study did not find any differences in affective responses between the IA and non-IA groups. Despite robust findings of higher depression and trait anxiety in addiction, mixed results have been obtained regarding acute affective response. Prior studies employing acute stress tasks reported that addiction was associated with more angry responses (e.g., Back et al., 2015; Romero-Martínez et al., 2019; Steinberg et al., 2011) but a normal level of general negative affect (e.g., Back et al., 2008; Ginty et al., 2012; Panknin et al., 2002). Also, IA is reportedly associated with an aggressive personality type (Gervasi et al., 2017) and a preference for violent digital games (Karaca et al., 2021). Therefore, the inconsistency between this and previous studies might be partly attributable to different affect types assessed between studies.
The current results of blunted cortisol response in IA are consistent with the accumulated evidence that addiction displayed blunted cortisol response. The results revealed extended blunted cortisol responses similar to those previously found in IGD and other IA addictions, further supporting the relationship between addiction and blunted cortisol response. Bibbey et al. (2015) reported that IA was associated with a normal cortisol response to a stressor. However, all the participants in their study, including those without IA, had lower cortisol responses, which might have caused difficulties in detecting differential responsivity. Moreover, the present study did not detect significant cortisol increase in IA, whereas Kaess et al. (2017) reported a slow but an increased cortisol response in IGD. This difference in cortisol responsivity might be due to the participants' sex ratio. All the participants in Kaess et al. (2017) were males, whereas a high ratio of females participated in the present study. It has been reported that males show higher cortisol response to a stressor than females (Liu et al., 2017). Another reason for the different results could be the different tasks used for stress induction between the two studies. Kaess et al. (2017) used the Trier social stress test (TSST), a standardized psychosocial stress task used for stress induction to evaluate neuroendocrine and cardiovascular stress responses. Therefore, the TSST might have elicited a more robust cortisol response.
The results of this study could imply that IA has a common vulnerability to stress with other addictions. Cortisol promotes carbohydrate, protein, lipid metabolization, immunosuppression, and anti-inflammatory effects. Therefore, cortisol secretion deficiency might inhibit these functions of cortisol during stressful experiences. It has been suggested that blunted cortisol responses reflect a suboptimal brain functioning in terms of motivational, endocrinal, and autonomic control regulation (Carroll et al., 2017). Neuroimaging studies in line with this suggestion have reported the hypoactivation of frontal and subcortical limbic regions associated with these functions in individuals with addictions and related conditions (Stice, 2008; Yang et al., 2015). In contrast, it has also been suggested that a blunted cortisol response might mitigate tissue damage caused by excessive cortisol exposure from chronic stress (Raffington et al., 2018). Thus, blunted cortisol might also have adaptive functions in addiction during prolonged stress.
The current results of normal SNS stress responses in IA are inconsistent with the prior findings of blunted SNS stress responses in individuals with addiction. Nonetheless, in contrast to the more robust results of blunted SNS responses in substance addiction, relatively inconsistent results of SNS stress responses were obtained in behavioral addiction; many studies have reported blunted SNS stress responses whereas some have reported normal responses (Maniaci et al., 2018). These mixed results may be, at least in part, due to the differences in ANS indexes used in the studies. Some prior studies used multiple ANS indexes and reported blunted heart rate (HR) and normal BP responses in addiction. For instance, individuals with gambling disorders show blunted HR and normal BP stress responses (Ginty et al., 2014; Heaney et al., 2011). A similar pattern of SNS stress response was also reported in alcoholism (Panknin et al., 2002; Sinha et al., 2009; Starcke et al., 2013). This differential sensitivity may be possible because HR change is dependent on SNS and parasympathetic nervous system activities, whereas BP change reflects SNS activities and blood vessel resistance (Armario et al., 2020). Moreover, as the indexes of SNS activities have differential sensitivity to stressor intensity (Armario et al., 2020) and coping used to address stress tasks (Florence et al., 2018; Obrist et al., 1978), they may have impacted the results.
This study has several limitations. First, the cross-sectional design of the study prevents us from proving a clear causal relationship. Second, because few studies have examined HPA and SNS stress responses in IA, the study was an explorative measure of a variety of factors (i.e., cortisol, alpha-amylase, and systolic and diastolic blood pressure). Furthermore, the lack of an a-priori sample size calculation could have led to a small sample size of individuals with IA. Thus, the present results should be considered preliminary, and future studies should test the replicability of the results, factoring in multiplicity adjustment and sample size calculation in advance. Third, the small sample size precludes an examination of sex difference in stress responses. Considering that sex was reported to modulate cortisol stress responses, this must be elucidated in future studies. Fourth, given that trait anxiety (e.g., Jezova et al., 2004) is reportedly related to a blunted cortisol response, this confounding factor could cause overestimating the relationship between IA and the blunted cortisol response. Future studies should test the independent contribution of IA to the blunted cortisol response.
In conclusion, this study suggests that IA is associated with a blunted cortisol response to a stressor, but further validation is needed. The results are consistent with prior findings in individuals with IGD and other addictions. This study implies that the stress system dysfunction for IA and other addictions are similar. Future studies should examine how these dysfunctional stress responses contribute to the development and relapse of IA.
Declarations
Author contribution statement
Hideki Tsumura: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mari Fukuda: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Hideyuki Kanda: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This study was supported by the Japan Society for the Promotion of Science [18K13349].
Data availability statement
The authors do not have permission to share data.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- al’Absi M., Ginty A.T., Lovallo W.R. Neurobiological mechanisms of early life adversity, blunted stress reactivity and risk for addiction. Neuropharmacology. 2021;188(September 2020) doi: 10.1016/j.neuropharm.2021.108519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpaslan A.H., Soylu N., Kocak U., Guzel H.I. Problematic Internet use was more common in Turkish adolescents with major depressive disorders than controls. Acta Paediatr. 2016;105(6):695–700. doi: 10.1111/apa.13355. [DOI] [PubMed] [Google Scholar]
- Armario A., Labad J., Nadal R. Focusing attention on biological markers of acute stressor intensity: empirical evidence and limitations. Neurosci. Biobehav. Rev. 2020;111(January):95–103. doi: 10.1016/j.neubiorev.2020.01.013. [DOI] [PubMed] [Google Scholar]
- Back S.E., Gros D.F., Price M., LaRowe S., Flanagan J., Brady K.T., Davis C., Jaconis M., McCauley J.L. Laboratory-induced stress and craving among individuals with prescription opioid dependence. Drug Alcohol Depend. 2015;155:60–67. doi: 10.1016/j.drugalcdep.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S.E., Waldrop A.E., Saladin M.E., Yeatts S.D., Simpson A., McRae A.L., Upadhyaya H.P., Contini Sisson R., Spratt E.G., Allen J., Kreek M.J., Brady K.T. Effects of gender and cigarette smoking on reactivity to psychological and pharmacological stress provocation. Psychoneuroendocrinology. 2008;33(5):560–568. doi: 10.1016/j.psyneuen.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardy N.C., King A.C., Lovallo W.R. Cardiovascular responses to physical and psychological stress in female alcoholics with transitory hypertension after early abstinence. Alcohol Clin. Exp. Res. 2003;27(9):1489–1498. doi: 10.1097/01.ALC.0000085587.00498.38. [DOI] [PubMed] [Google Scholar]
- Bibbey A., Phillips A.C., Ginty A.T., Carroll D. Problematic Internet use, excessive alcohol consumption, their comorbidity and cardiovascular and cortisol reactions to acute psychological stress in a student population. J. Behav. Addict. 2015;4(2):44–52. doi: 10.1556/2006.4.2015.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand L.A., Blendy J.A. Molecular and genetic substrates linking stress and addiction. Brain Res. 2010;1314:219–234. doi: 10.1016/j.brainres.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel A.W. Tobacco addiction and the dysregulation of brain stress systems. Neurosci. Biobehav. Rev. 2012;36(5):1418–1441. doi: 10.1016/j.neubiorev.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D., Ginty A.T., Whittaker A.C., Lovallo W.R., de Rooij S.R. The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neurosci. Biobehav. Rev. 2017;77:74–86. doi: 10.1016/j.neubiorev.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Hollunder B., Garbusow M., Sebold M., Heinz A. The physiological responses to acute stress in alcohol-dependent patients: a systematic review. Eur. Neuropsychopharmacol. 2020;41:1–15. doi: 10.1016/j.euroneuro.2020.09.003. [DOI] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Donovan B., Padin-Rivera E., Kowaliw S. Transcend”: initial outcomes from a posttraumatic stress disorder/substance abuse treatment program. J. Trauma Stress. 2001;14(4):757–772. doi: 10.1023/A:1013094206154. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H., Schuck J., Stender N., Pilz J., Gefeller O., Schilling L., Poser W., Kaw S. Endocrine and hemodynamic effects of stress versus systemic CRF in alcoholics during early and medium term abstinence. Alcohol Clin. Exp. Res. 1997;21(7):1285–1293. [PubMed] [Google Scholar]
- Florence T.J., Kent de Grey R.G., Uchino B.N., Cronan S. A longitudinal analysis of coping style and cardiovascular reactivity to laboratory stressors. Pers. Indiv. Differ. 2018;125(January):112–115. [Google Scholar]
- Gerra G., Somaini L., Manfredini M., Raggi M.A., Saracino M.A., Amore M., Leonardi C., Cortese E., Donnini C. Dysregulated responses to emotions among abstinent heroin users: correlation with childhood neglect and addiction severity. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2014;48:220–228. doi: 10.1016/j.pnpbp.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Gervasi A.M., La Marca L., Costanzo A., Pace U., Guglielmucci F., Schimmenti A. Personality and internet gaming disorder: a systematic review of recent literature. Curr. Addict. Rep. 2017;4(3):293–307. [Google Scholar]
- Ginty A.T., Jones A., Carroll D., Roseboom T.J., Phillips A.C., Painter R., de Rooij S.R. Neuroendocrine and cardiovascular reactions to acute psychological stress are attenuated in smokers. Psychoneuroendocrinology. 2014;48:87–97. doi: 10.1016/j.psyneuen.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Ginty A.T., Phillips A.C., Higgs S., Heaney J.L.J., Carroll D. Disordered eating behaviour is associated with blunted cortisol and cardiovascular reactions to acute psychological stress. Psychoneuroendocrinology. 2012;37(5):715–724. doi: 10.1016/j.psyneuen.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Goeders N.E. The impact of stress on addiction. Eur. Neuropsychopharmacol. 2003;13(6):435–441. doi: 10.1016/j.euroneuro.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Heaney J.L.J., Ginty A.T., Carroll D., Phillips A.C. Preliminary evidence that exercise dependence is associated with blunted cardiac and cortisol reactions to acute psychological stress. Int. J. Psychophysiol. 2011;79(2):323–329. doi: 10.1016/j.ijpsycho.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Iwahashi S., Tanaka Y., Fukudo S., Hongo M. The development of the Japanese version of the perceived stress scale. Jpn. J. Psychosom. Med. 2002;42:459–466. [Google Scholar]
- Jezova D., Makatsori A., Duncko R., Moncek F., Jakubek M. High trait anxiety in healthy subjects is associated with low neuroendocrine activity during psychosocial stress. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2004;28(8):1331–1336. doi: 10.1016/j.pnpbp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Kaess M., Parzer P., Mehl L., Weil L., Strittmatter E., Resch F., Koenig J. Stress vulnerability in male youth with internet gaming disorder. Psychoneuroendocrinology. 2017;77:244–251. doi: 10.1016/j.psyneuen.2017.01.008. [DOI] [PubMed] [Google Scholar]
- Karaca A., Demirci N., Caglar E., Konsuk Unlu H. Correlates of Internet addiction in Turkish adolescents. Child. Youth Serv. Rev. 2021;126(November 2020) [Google Scholar]
- Kuss D.J., Kristensen A.M., Lopez-Fernandez O. Internet addictions outside of Europe: a systematic literature review. Comput. Hum. Behav. 2021;115(November 2020) [Google Scholar]
- Levin Y., Lev Bar-Or R., Forer R., Vaserman M., Kor A., Lev-Ran S. The association between type of trauma, level of exposure and addiction. Addict. Behav. 2021;118(November 2020) doi: 10.1016/j.addbeh.2021.106889. [DOI] [PubMed] [Google Scholar]
- Liu J.J.W., Ein N., Peck K., Huang V., Pruessner J.C., Vickers K. Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): a meta-analysis. Psychoneuroendocrinology. 2017;82:26–37. doi: 10.1016/j.psyneuen.2017.04.007. October 2016. [DOI] [PubMed] [Google Scholar]
- Maniaci G., Goudriaan A.E., Cannizzaro C., van Holst R.J. Impulsivity and stress response in pathological gamblers during the trier social stress test. J. Gambl. Stud. 2018;34(1):147–160. doi: 10.1007/s10899-017-9685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrist P.A., Gaebelein C.J., Teller E.S., Langer A.W., Grignolo A., Light K.C., McCubbin J.A. The relationship among heart rate, caratid dP/dt, and blood pressure in humans as a function of the type of stress. Psychophysiology. 1978;15(2):102–115. doi: 10.1111/j.1469-8986.1978.tb01344.x. [DOI] [PubMed] [Google Scholar]
- Osada H. Internet addiction in Japanese college students: is Japanese version of Internet Addiction Test (JIAT) useful as a screening tool? Bull. Senshu Univ. Sch. Human Sciences. 2013;3(1):71–80. [Google Scholar]
- Panknin T.L., Dickensheets S.L., Nixon S.J., Lovallo W.R. Attenuated heart rate responses to public speaking in individuals with alcohol dependence. Alcohol Clin. Exp. Res. 2002;26(6):841–847. [PubMed] [Google Scholar]
- Paris J.J., Franco C., Sodano R., Frye C.A., Wulfert E. Gambling pathology is associated with dampened cortisol response among men and women. Physiol. Behav. 2010;99(2):230–233. doi: 10.1016/j.physbeh.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prizant-Passal S., Shechner T., Aderka I.M. Social anxiety and internet use - a meta-analysis: what do we know? What are we missing? Comput. Hum. Behav. 2016;62:221–229. [Google Scholar]
- Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Raffington L., Prindle J., Keresztes A., Binder J., Heim C., Shing Y.L. Blunted cortisol stress reactivity in low–income children relates to lower memory function. Psychoneuroendocrinology. 2018;90:110–121. doi: 10.1016/j.psyneuen.2018.02.002. December 2017. [DOI] [PubMed] [Google Scholar]
- Romero-Martínez Á., Vitoria-Estruch S., Moya-Albiol L. Emotional and autonomic dysregulation in abstinent alcoholic men: an idiosyncratic profile? Alcohol. 2019;77:155–162. doi: 10.1016/j.alcohol.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Sato A., Yasuda A. Development of the Japanese version of positive and negative affect schedule (PANAS) scales. Jpn. J. Pers. 2001;9:138–139. (in Japanese) [Google Scholar]
- Saxon A.J., Davis T.M., Sloan K.L., McKnight K.M., McFall M.E., Kivlahan D.R. Trauma, symptoms of posttraumatic stress disorder, and associated problems among incarcerated veterans. Psychiatr. Serv. 2001;52(7):959–964. doi: 10.1176/appi.ps.52.7.959. [DOI] [PubMed] [Google Scholar]
- Shima S., Shikano T., Kitamura T., Asai M. New self-rating scales for depression. Seishin Igaku. 1985;27:717–723. (In Japanese) [Google Scholar]
- Shimizu H. An introduction to the statistical free software HAD: suggestions to improve teaching, learning and practice data analysis. J. Media Inform. Commun. 2016;1:59–73. (in Japanese) [Google Scholar]
- Shimizu H., Imae K. Development of the Japanese edition of the spielberger state-trait anxiety inventory (STAI) for student use. Jpn. J. Educ. Psychol. 1981;29:62–67. (In Japanese.) [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Fox H.C., Hong K.A., Bergquist K., Bhagwagar Z., Siedlarz K.M. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34(5):1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Jastreboff A.M. Stress as a common risk factor for obesity and addiction. Biol. Psychiatr. 2013;73(9):827–835. doi: 10.1016/j.biopsych.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R.E. Consulting Psychologists Press; Palo Alto, CA: 1997. Manual for the State-Trait Anxiety Inventory (Self-evaluation Questionnaire) [Google Scholar]
- Starcke K., van Holst R.J., van den Brink W., Veltman D.J., Goudriaan A.E. Physiological and endocrine reactions to psychosocial stress in alcohol use disorders: duration of abstinence matters. Alcohol Clin. Exp. Res. 2013;37(8):1343–1350. doi: 10.1111/acer.12103. [DOI] [PubMed] [Google Scholar]
- Steinberg L., Tremblay A.M., Zack M., Busto U.E., Zawertailo L.A. Effects of stress and alcohol cues in men with and without problem gambling and alcohol use disorder. Drug Alcohol Depend. 2011;119(1–2):46–55. doi: 10.1016/j.drugalcdep.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Stice E., Spoor S., Bohon C., Veldhuizen M.G., Small D.M. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J. Abnorm. Psychol. 2008;117(4):924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno M., Teo A.R., Shiraishi M., Tayama M., Kawanishi C., Kato T.A. Prevalence rate of Internet addiction among Japanese college students: two cross-sectional studies and reconsideration of cut-off points of Young’s Internet Addiction Test in Japan. Psychiatr. Clin. Neurosci. 2018;72(9):723–730. doi: 10.1111/pcn.12686. [DOI] [PubMed] [Google Scholar]
- Tsumura H., Kanda H., Sugaya N., Tsuboi S., Fukuda M., Takahashi K. Problematic internet use and its relationship with psychological distress, insomnia, and alcoholism among schoolteachers in Japan. Cyberpsychol., Behav. Soc. Netw. 2018;21(12):788–796. [Google Scholar]
- Turner R.J., Lloyd D.A. Cumulative adversity and drug dependence in young adults: racial/ethnic contrasts. Addiction. 2003;98(3):305–315. doi: 10.1046/j.1360-0443.2003.00312.x. [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weinstein A., Feder L.C., Rosenberg K.P., Dannon P. In: Behavioral Addictions. Rosenberg K.P., Feder L.C., editors. Academic Press; San Diego, CA: 2014. Internet addiction disorder: overview and controversies; pp. 99–117. [Google Scholar]
- Widyanto L., McMurran M. The psychometric properties of the internet addiction test. Cyberpsychol. Behav.: Impact Internet Multimedia Virt. Real. Behav. Soc. 2004;7(4):443–450. doi: 10.1089/cpb.2004.7.443. [DOI] [PubMed] [Google Scholar]
- Yang H., Spence J.S., Briggs R.W., Rao U., North C., Devous M.D., Xiao H., Adinoff B. Interaction between early life stress and alcohol dependence on neural stress reactivity. Addiction Biol. 2015;20(3):523–533. doi: 10.1111/adb.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. John Wiley & Sons; New York: 1998. Caught in the Net. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.