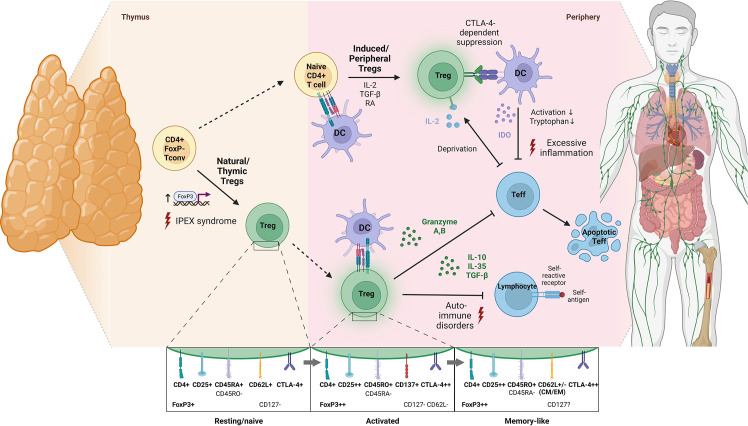
Figure 1.
Schematic illustration of Treg development, modes of activation and physiological functions. According to an affinity-based model of T cell development, a fraction of self-reactive T cells with intermediate affinity for self-peptide-MHC complexes differentiates into natural Tregs by upregulation of FoxP3 and CD25 in response to T cell receptor (TCR) signaling (thymus-derived Tregs; ~80% of peripheral Treg repertoire) (31). Also, antigenic stimulation of peripheral naïve T cells in the presence of TGF-β, IL-2 and retinoic acid induces their differentiation into FoxP3+ Tregs (peripherally-derived Tregs) (28). Of note, phenotypical transition of Tregs upon activation is not associated with de novo expression of surface proteins but rather with quantitative shifts in the expression levels of molecules already expressed in a resting state. Specifically, upon TCR activation, Tregs downregulate CD45RA and upregulate CD45RO, CD25, CTLA-4 and FoxP3 (32, 33). In contrast to other T cell subsets, the transition of Tregs into a stable memory pool after antigen elimination is still debated and so far, only a few marker candidates such as CD62L have been proposed for identification of memory Tregs (34). Functionally, Tregs are involved in suppressing immune responses towards self and non-self-antigens via inhibitory cytokine secretion, granzyme-dependent and cytokine-deprivation-mediated effector cell inhibition as well as cell-contact-dependent alteration of dendritic cell function and maturation (potential consequences of deficient Treg development or function are indicated by red lightnings) (35) (created with BioRender.com). CM, central memory; CTLA-4, cytotoxic T lymphocyte antigen 4; DC, dendritic cell; EM, effector memory; IDO, indoleamine 2,3-dioxygenase; IPEX, Immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome; RA, retinoic acid.