FIGURE 1.
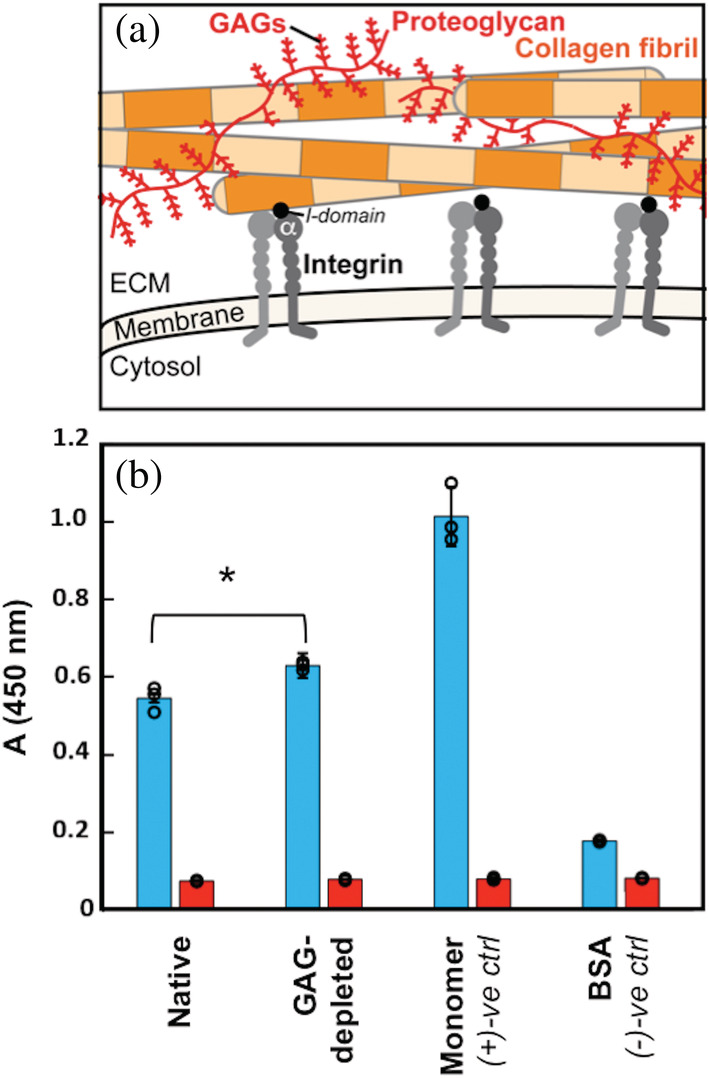
Collagen I fibril–integrin α2I interactions in the native ECM environment. (a) Cartoon highlighting multiple components of the ECM. In this study, we focus on collagen I fibrils (orange); integrin cell surface receptors (gray), specifically the I‐domain (black) of the α‐subunit (αI); and glycosaminoglycans (GAGs), which are polysaccharide chains displayed on proteoglycans (red). Integrin αI domains and GAGs interact with individual collagen I fibrils in the ECM. (b) ELISA binding assay of integrin α2I (10 μg/ml) binding to collagen I in varying contexts (native: collagen I fibrils from rat tail tendon ECM in phosophate buffered saline (PBS), GAG‐depleted: collagen I fibrils from ECM with an estimated 85% GAG depletion in PBS, monomer: purified collagen I from rat tail tendon in 10 mM acetic acid, and BSA: 50 mg/mL bovine‐serum albumin in PBS). A one‐way ANOVA analysis confirmed that there is a significant difference between α2I binding to native and GAG‐depleted collagen I fibrils (15% increase for GAG‐depleted collagen I fibrils) with a p‐value of <0.05 (*). Each binding condition was performed in the presence of either 5 mM MgCl2 (blue) or 5 mM EDTA (red). Error bars indicate the standard deviation of the measurements, which were taken in triplicate. Individual measurements are shown as circles. ECM, extracellular matrix
