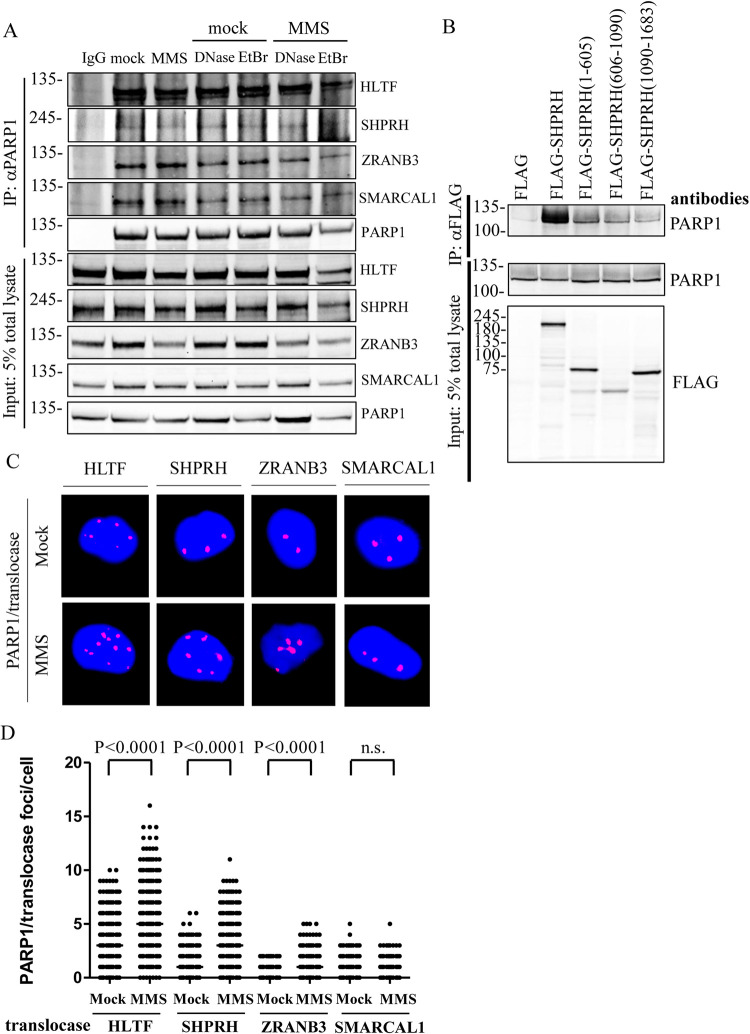
Fig 3. PARP1 interacts with DNA translocases in vivo.
(A) The endogenous protein–protein interaction between PARP1 and translocases. Cells were treated with either mock or 0.01% MMS for 1 hour. Cell lysates were treated with either 100 μg/ml DNase I or 50 μg/ml EtBr as indicated. The endogenous PARP1 was immunoprecipitated with an anti-PARP1 antibody and the pulldown translocases were detected with specific antibodies as indicated. The non-specific mouse IgG was used as a negative control. Input represents 5% of total cell lysates. (B) The N-terminal domain of SHPRH interacts with PARP1. HEK293T cells were transfected with various FLAG–SHPRH constructs. The FLAG-SHPRH fusion proteins were immunoprecipitated with anti-FLAG sepharose beads, and the immunoprecipitates were then subjected to immunoblotting analysis. Input represents 5% of total cell lysates. (C) Representative images of HLTF, SHPRH, ZRANB3, and SMARCAL1/PARP1-PLA foci in T24 cells, respectively. Cells were treated with 0.01% MMS for 1 hour. The association of each translocase with PARP1 was determined by the PLA assay. (D) Distributions of HLTF, SHPRH, ZRANB3, and SMARCAL1/PARP1-PLA foci derived from C. At least 200 cells from each condition were measured. (Raw PLA data in S3 Data).