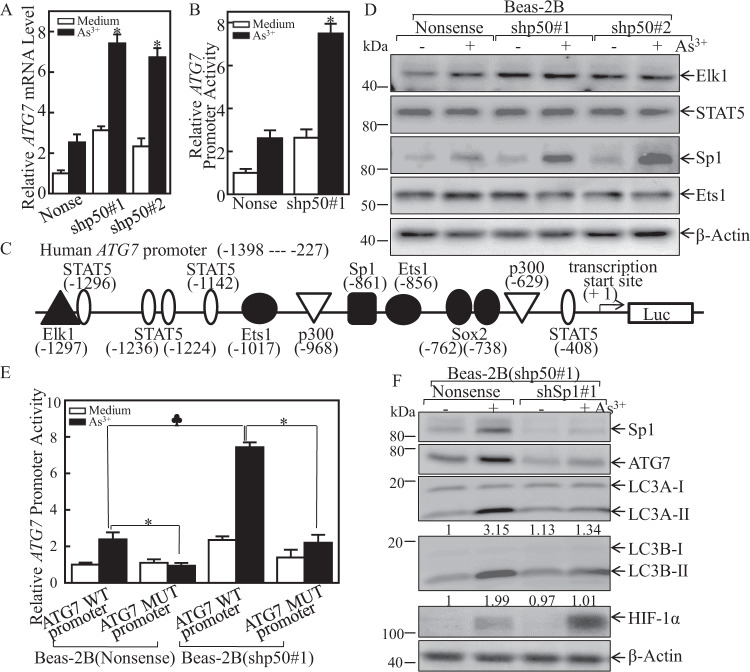
Fig. 4. p50 attenuated ATG7 transcription and expression by inhibition of Sp1 expression following arsenite treatment.
A Beas-2B(Nonsense) and Beas-2B(shp50) cells were treated with 20 μM arsenite and the ATG7 mRNA expression was determined by Real-Time PCR assay; The symbol (*) indicates a significant increase as comparison to WT cells treated with arsenite (p < 0.05). B The ATG7 promoter-driven luciferase reporter was transfected into Beas-2B(Nonsense) and Beas-2B(shp50) cells. The stable transfectants were subjected to 20 μM arsenite treatment for 12 h, and the cell extracts were subjected to luciferase activity assay to evaluate relative ATG7 promoter transcriptional activity. The bars show mean ± SD from 3 independent experiments. The symbol (*) indicates a significant increase as comparison to WT cells treated with arsenite (p < 0.05). C Potential transcription factor binding sites in the ATG7 promoter region (−1398 to −227) were analyzed using the TRANSFAC 8.3 engine online. D Beas-2B(Nonsense) and Beas-2B(shp50) cells were treated with 20 μM arsenite for 24 h. The cells were extracted and cell lysates were subjected to western blot by using the indicated antibodies. E Wild-type or mutant ATG7 promoter-driven luciferase reporters was co-transfected together with pRL-TK into Beas-2B(Nonsense) and Beas-2B(shp50) cells. 24 h post-transfection, the transfectants were extracted to evaluate the luciferase activity. The results were presented as relative ATG7 promoter activity in response to arsenite exposure. Each bar indicates mean ± SD from 3 independent experiments. The asterisk (*) indicates a significant decrease compared with wild-type reporter transfectant (p < 0.05). The spade (♣) indicates a significant increase compared with Beas-2B(Nonsense) cells (p < 0.05). F The stable transfectants as indicated were exposed to 20 μM arsenite for 24 h. Cell extracts were subjected to Western blot, the ratio of LC3 II/LC3 I was quantified.