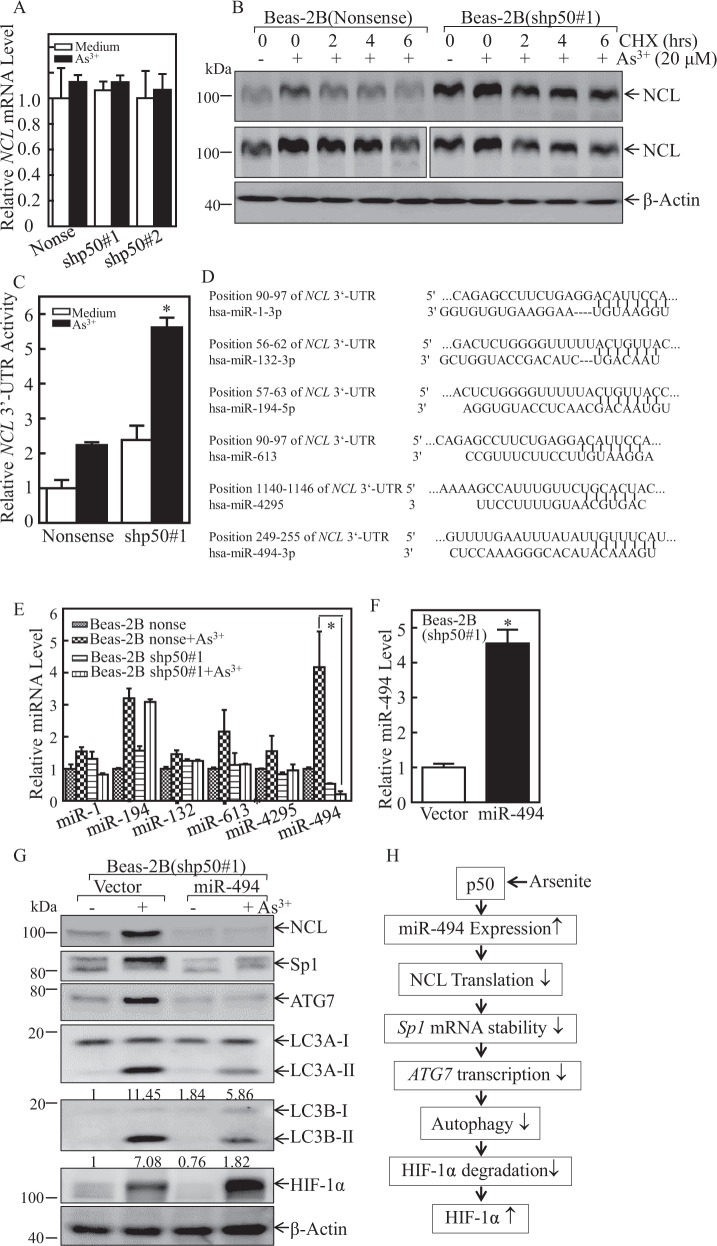
Fig. 6. p50-mediated MiR-494 upregulation directly binding to NCL 3’-UTR and attenuated its protein translation and expression upon arsenite treatment.
A Beas-2B(Nonsense) and Beas-2B(shp50) cells were treated with 20 μM arsenite and Real-Time PCR assay was performed to evaluate the NCL mRNA expression. B Beas-2B(Nonsense) and Beas-2B(shp50) cells were pretreated with arsenite (20 μM) for 12 h and then exposed to CHX (10 μM) for the indicated time points after the removal of arsenite. The cell extracts were subjected to western blot for determine NCL protein levels. C Wild-type NCL 3’-UTR-drived luciferase reporters together with pRL-TK were co-transfected into Beas-2B(Nonsense) and Beas-2B(shp50) cells, respectively. The transfectants were treated with arsenite for 24 h, and cell extracts were subjected to the luciferase assay. The results were presented as NCL 3’-UTR activity relative to the vector control transfectant, and each bar indicates mean ± SD from 3 independent experiments. D The potential miRNA binding sites in the 3’-UTR region of NCL mRNA. E Real-time PCR was used to determine the expression levels of miRNA in Beas-2B(Nonsense) and Beas-2B(shp50) cells following arsenite exposure. F Real-time PCR was performed to evaluate the miR-494 expression in Beas-2B(shp50#1/miR-494) transfectant in comparison to that in Beas-2B(shp50#1/Vector) cells. G The indicated stable transfectants were treated with arsenite for 24 h and cell extracts were subjected to western blot for evaluating the effect of miR-494 on expression of NCL, Sp1, ATG7, HIF-1α and the conversion of LC3 from LC3-I to LC3-II. β-Actin was used as a protein loading control, the ratio of LC3 II/LC3 I was quantified. H molecular mechanisms underlying p50 promotion of HIF-1α protein accumulation through suppression of ATG7-dependent autophagy.