Figure 3.
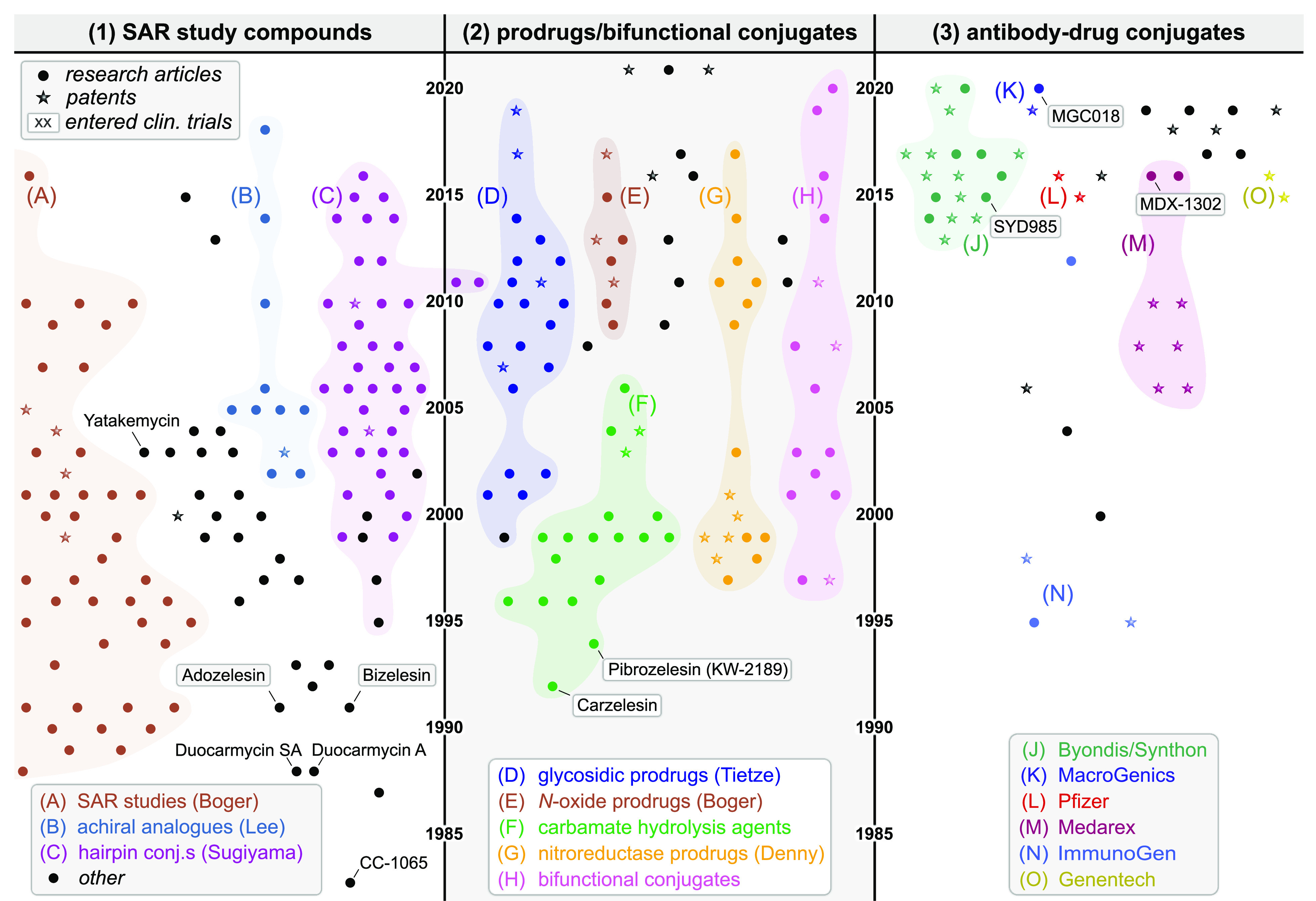
Structural developments of the duocarmycins (cartoon; all chemical structures in Poster S1). In Group 1 (SAR compounds), studies resolved the molecular motifs crucial for rational tuning of bioactivity. In Group 2 (Prodrugs), non-natural prodrugs (glycosides, nitroaryls, carbamates, N-oxides) and bifunctional conjugates expanded the scope of duocarmycins. In Group 3 (ADCs), industry has been a main driver of research.
