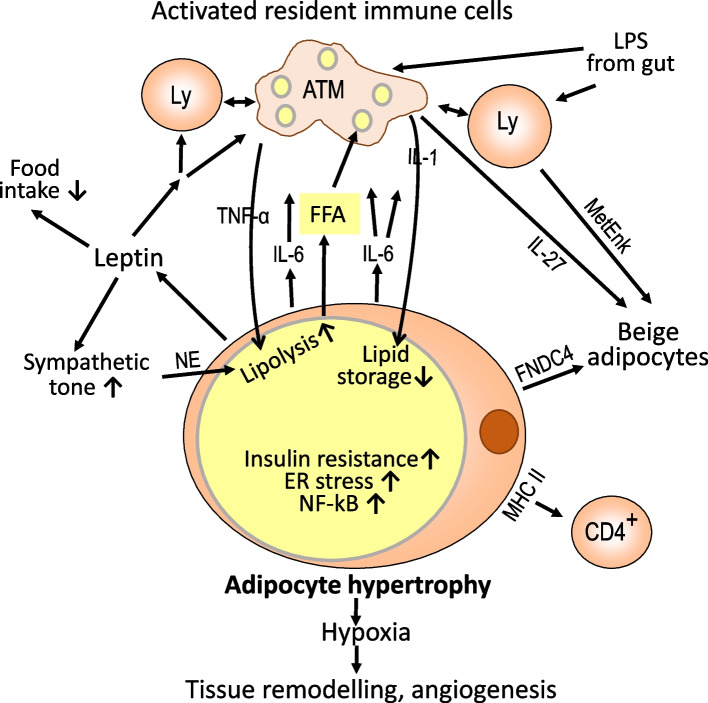
Fig. 3.
Local inflammation in response to disturbed adipocyte metabolic homeostasis. When enhanced lipid storage via adipocyte enlargement and differentiation of progenitor cells fails to maintain metabolic homeostasis, local inflammatory changes occur in order to dispose of excess lipid and regain metabolic control. For one, lipid-laden adipocytes experience endoplasmic reticulum stress and increased expression of NFkB leading to the release of pro-inflammatory mediators such as IL-6. Additional pro-inflammatory signals are delivered by the release of free fatty acids, leptin, lipopolysaccharides, and other products of an unbalanced microbiota in the context of a leaky gut. Activated resident immune cells release amounts of pro-inflammatory mediators sufficient to promote lipolysis and suppress lipid storage in part via induction of insulin resistance. In addition, there is an uptake of lipids by macrophages and storage in small lipid droplets. Leptin interacts with receptors in the brain to limit food intake and increase the sympathetic tone. The increased local release of noradrenaline also promotes lipolysis. Another pro-inflammatory condition results from hypoxia due to local enlargement of adipocytes. The concomitant release of enzymes and factors promoting tissue remodeling and angiogenesis may be considered a healing response. Enlarged adipocytes overexpress MHC class II antigens and appear to present antigens to CD4-positive T cells. Another pathway of limiting energy storage is the induction of adipocyte beiging by transdifferentiation or growth from progenitors and the disposal of excess energy by thermogenesis. For better readability, only a few key intercellular signals are included in the scheme. FNDC4, fibronectin type III domain-containing protein 4; FFA, free fatty acids; LPS, lipopolysaccharide; NE, norepinephrine/noradrenaline; NFkB, nuclear factor kappa B