Fig. 1. Elastic fiber formation and degradation.
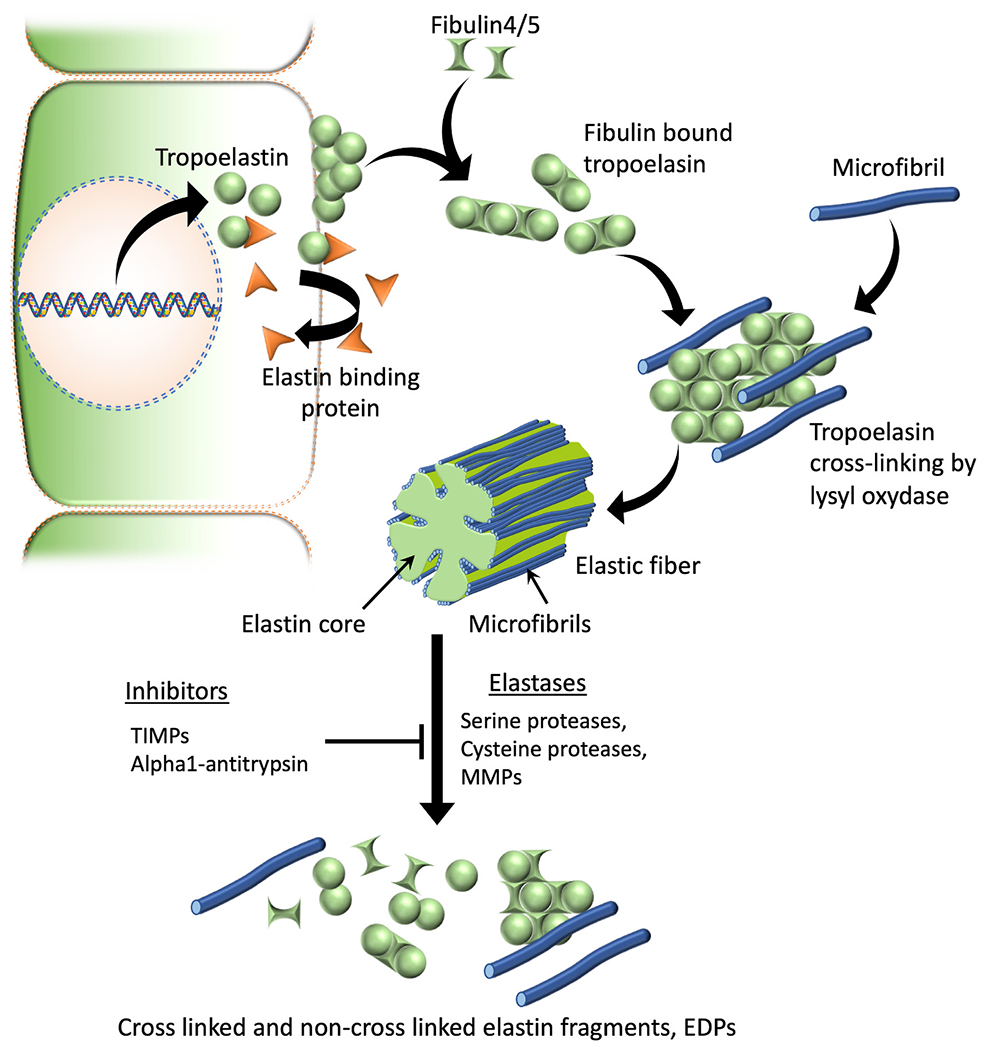
Tropoelastin monomers are formed intracellularly, which then will be chaperoned and transported to the ECM by elastin binding proteins. At the cell surface elastin binding proteins will be released leading to tropoelastin self-aggregation or coacervation. Coacervation leads to next stages of elastic fiber assembly such as binding of fibulins, microfibrillar deposition, recruitment of lysyl oxidase, elastin cross-linking and mature fiber formation. Serine proteases such as neutrophil elastase & HTRA1, cysteine proteases like cathepsins K, V, L, S & B, and several metalloproteases (MMPs 2, 3, 7, 9, 12 & 14) are known to have elastolytic capability and can contribute to the degradation of insoluble elastic fibers to generate elastin fragments or EDPs. TIMPs and alpha-1 proteases such as alpha-1 antitrypsin are known elastase inhibitors that can prevent the degradation of elastic fibers.
Abbreviations: TIMPs, Tissue inhibitors of metalloproteinases; MMPs, Matrix metalloproteinases; EDPs, Elastin-derived peptides.
