Summary
We explore needle sugar isotopic compositions (δ18O and δ13C) in boreal Scots pine (Pinus sylvestris) over two growing seasons.
A leaf‐level dynamic model driven by environmental conditions and based on current understanding of isotope fractionation processes was built to predict δ18O and δ13C of two hierarchical needle carbohydrate pools, accounting for the needle sugar pool size and the presence of an invariant pinitol pool.
Model results agreed well with observed needle water δ18O, δ18O and δ13C of needle water‐soluble carbohydrates (sugars + pinitol), and needle sugar δ13C (R 2 = 0.95, 0.84, 0.60, 0.73, respectively). Relative humidity (RH) and intercellular to ambient CO2 concentration ratio (C i/C a) were the dominant drivers of δ18O and δ13C variability, respectively. However, the variability of needle sugar δ18O and δ13C was reduced on diel and intra‐seasonal timescales, compared to predictions based on instantaneous RH and C i/C a, due to the large needle sugar pool, which caused the signal formation period to vary seasonally from 2 d to more than 5 d. Furthermore, accounting for a temperature‐sensitive biochemical 18O‐fractionation factor and mesophyll resistance in 13C‐discrimination were critical.
Interpreting leaf‐level isotopic signals requires understanding on time integration caused by mixing in the needle sugar pool.
Keywords: boreal forest, carbon isotope, dynamic modeling, needle sugar, oxygen isotope, photosynthesis, Scots pine (Pinus sylvestris)
Short abstract
See also the Commentary on this article by Ubierna et al., 236: 2003–2008.
Introduction
Stable carbon and oxygen isotope compositions in tree rings (δ13C and δ18O, respectively) provide records of past environmental and tree physiological signals (McCarroll & Loader, 2004; Battipaglia et al., 2013). Tree ring δ13C and δ18O records are foreseen as powerful tools in advancing our understanding on the response of forests to changing climate and increasing atmospheric CO2 concentration (Gessler et al., 2014). The formation of tree ring δ13C and δ18O signals in boreal coniferous species is of special interest, because these sensitive ecosystems play a critical role in the carbon cycle of our planet (Snyder et al., 2004) and are now undergoing climatic warming at a significantly faster rate compared to the global average (IPCC, 2021). To this end, we need to understand how isotopic signals are formed in the leaves, where most of the interaction with local environmental conditions occurs. Even though, this is a vastly studied field, intra‐seasonal studies in boreal forest are scarce.
Newly assimilated photosynthates are products of atmospheric CO2 and leaf water, thus reflecting δ13C of atmospheric CO2 strongly modified by leaf biochemistry (Farquhar et al., 1982) and δ18O of source water that is further 18O‐enriched in leaves during evaporation (Farquhar & Lloyd, 1993). 18O‐enrichment due to evaporation is linked to the variation of relative humidity (RH) as defined by the model of Craig & Gordon (1965), developed for open water bodies and adapted later to leaves (Dongmann et al., 1974). The Craig–Gordon model is, however, prone to overestimate 18O‐enrichment (Cernusak et al., 2016), which has led to the introduction of the Péclet effect (Farquhar & Lloyd, 1993; Barbour et al., 2000) and the two‐pool concept (Leaney et al., 1985; Roden et al., 2015). The δ18O of leaf photosynthates is expected to reflect the δ18O of leaf water with an offset of 27‰ (Barbour et al., 2000; Cernusak et al., 2003). Although the offset 27‰ is commonly assumed constant, Sternberg & Ellsworth (2011) reported it to be inversely related to temperature, with a particularly strong incline below 20°C. Recently, Hirl et al. (2021) acknowledged the need to account for this temperature dependence, when modeling the seasonal variation of cellulose δ18O in a grassland ecosystem, underlining the power of modeling in testing our current theoretical understanding. In cooler boreal conditions, such temperature dependence is potentially of even higher importance.
Regarding δ13C, the pathway to primary photosynthates in C3 plants can be divided into fractionation by diffusion from air to chloroplast through stomata and mesophyll, and biochemical fractionation, that is, carboxylation, mitochondrial respiration and photorespiration (Farquhar et al., 1982; Cernusak et al., 2013). Recent studies have emphasized the importance of dynamic mesophyll conductance on δ13C of assimilates (Stangl et al., 2019; Schiestl‐Aalto et al., 2021). Mechanistic modeling studies have further shown that tree ring δ13C signals are highly sensitive to isotopic fractionation during both photosynthesis and respiration (Ogée et al., 2009; Eglin et al., 2010) and that the sugars used for leaf respiration may have a δ13C value that differs from current assimilates (Wingate et al., 2007). These findings together with the fact that the fractionation factors of these processes are still poorly constrained in vivo (Barbour & Song, 2014), complicate modeling δ13C signals in plants.
Interpreting and predicting isotopic signals in leaves may be further complicated in field conditions with strong variability in environmental conditions over sub‐daily to seasonal timescales. For example, leaf water δ18O sampled on sub‐daily timescales in varying ambient conditions has been shown to deviate significantly from values predicted by a steady‐state model (Farquhar & Cernusak, 2005; Gessler et al., 2013). Similar nonsteady‐state effects are expected, when predicting the isotopic composition of leaf sugars as the sugar pool does not represent only the current assimilates (Barnard et al., 2007; Gessler et al., 2013). There are dynamic models describing intra‐seasonal variation in δ13C and δ18O in tree ring cellulose (e.g. Ogée et al., 2009; Eglin et al., 2010), but similar descriptions for leaf sugar pools are scarce. These earlier studies face the difficulty of differentiating between fractionation processes in the leaves and during the pathway to tree rings, which stresses the need for leaf‐level mechanistic models.
Measurements of the isotopic composition of leaf sugars is challenging in many respects. Primarily, studies often analyze only mixtures of compounds (e.g. total leaf organic matter, water‐soluble organic matter, or purified water‐soluble extracts), which tends to reduce the temporal variability of the isotopic signal of interest, as compounds within the mixture can have different live‐spans in the leaf and differ in their isotope value due to fractionations during secondary metabolism (Barnard et al., 2007; Offermann et al., 2011; Gessler et al., 2013; Rinne et al., 2015; Lehmann et al., 2020). Purified water extracts (hereafter water‐soluble carbohydrates, WSC) are expected to consist mainly of sugars (Richter et al., 2009; Rinne et al., 2015) and have therefore potentially more variable isotopic signal than total organic matter. However, if sugar alcohols (e.g. pinitol/myo‐inositol, hereafter referred to as pinitol; see Rinne et al., 2012) are present in high concentrations, they have been shown to reduce the variability of the δ13C signal of WSC (Richter et al., 2009; Rinne et al., 2015). Permanent high fraction of pinitol throughout the growing season is typical for conifer needles in high‐latitude and high‐elevation conditions (Lipavská et al., 2000; Streit et al., 2013; Rinne et al., 2015). To address this issue, compound‐specific isotope analysis (CSIA) of δ13C provides means to retrieve the isotopic signal of e.g. sucrose, the main transport sugar, from the bulk sample (Rinne et al., 2012). For δ18O, the analytical process is more laborious and only a few studies have reported CSIA results for a limited number of samples (Lehmann et al., 2016, 2017). Intra‐seasonal CSIA of δ18O are therefore still out of reach for modeling purposes. However, making use of the CSIA results of δ13C, compound concentrations, and concurrently analyzed δ13C and δ18O of WSC, mechanistic models can attempt to answer how variable δ18O is between various compounds.
This study explores the formation of needle sugar isotopic signals in boreal Scots pine over two growing seasons in southern Finland. We concurrently present observations and environmentally driven dynamic modeling of the intra‐seasonal variation of δ18O and δ13C of needle WSC. Our isotopic data consists of δ18O in water pools (e.g. twig and needle), δ18O and δ13C analyzed from WSC as bulk, and δ13C results from CSIA. Combining mechanistic modeling and data, we aim to address the following research questions:
Which fractionation and mixing processes are important for capturing intra‐seasonal variation of needle sugar δ18O and δ13C?
What is the role of the sugar pool size in predicting needle sugar δ18O and δ13C?
What implications do needle sugar pool size and the composition of bulk samples have on interpreting environmental/physiological signals from needle δ18O and δ13C?
Model description
The built model describes the dynamics of δ18O and δ13C in two hierarchical needle carbohydrate pools in response to half‐hourly environmental conditions (air temperature, photosynthetic active radiation (PAR), vapor pressure, atmospheric CO2 concentration, and soil moisture) and isotopic input data (δ18O of water vapor and source water, and δ13C of atmospheric CO2). The two carbohydrate pools described in the model are needle: (1) sugars; and (2) WSC, which in addition to sugars contain pinitol. The model builds on a photosynthesis model solving leaf net CO2 exchange, including carboxylation, photorespiration, and mitochondrial respiration (see Supporting Information Methods S1; Table S1). Doing so it accounts for stomatal control and mesophyll resistance, but neglects leaf energy balance assuming the leaf is at air temperature (a fair assumption, given the small size of Scots pine needles that are well‐coupled to the atmosphere; Launiainen et al., 2016; Kim et al., 2018). Theory‐based isotopic fractionation of oxygen and carbon in needles and their accumulation in the needle sugar and WSC pools are then solved as described in the following section. Model equations are expressed in terms of isotopic ratios (R), which can be converted to ‘delta’ notations:
| (Eqn 1) |
where R std is the isotope ratio of an international standard; Vienna Standard Mean Ocean Water (VSMOW) for 18O/16O and Vienna Peedee belemnite (VPDB) for 13C/12C.
Formation of needle sugar δ18O
In steady‐state conditions, the isotopic ratio of oxygen at evaporative sites is given by Flanagan et al. (1991)
| (Eqn 2) |
where R s and R v are the isotopic ratios of oxygen in source water and atmospheric water vapor, respectively; α + (= 1 + ε +) is the temperature‐dependent equilibrium fractionation during vaporization (Majoube, 1971); w a and w i (in mol mol−1) are the mole fractions of water vapor in the atmosphere and inside the leaf, respectively; and α k is the kinetic isotope fractionation during water vapor diffusion through stomata and leaf boundary layer:
| (Eqn 3) |
where g s and g b (in mol m−2 s−1) are stomatal and boundary layer conductances for CO2 (assuming both scale for water vapor by 1.6); and ε ks and ε kb are the fractionation factors associated with the diffusion through stomata and the boundary layer, respectively (Table 1).
Table 1.
Parameters for isotopic modeling.
| Parameter a | Description | Value | Source |
|---|---|---|---|
| (−) | Fractionation during diffusion of CO2 through the boundary layer | 2.9‰ | Farquhar (1983) |
| (−) | Fractionation during diffusion of CO2 through stomata | 4.4‰ | O'Leary (1981) |
| (−) | Fractionation during transfer of CO2 through mesophyll | 1.8‰ | O'Leary (1984) |
| b (−) | Fractionation during caboxylation | 29‰ | Roeske & O'Leary (1984) |
| f (−) | Fractionation during photorespiration | 8‰ | Ghashghaie et al. (2003) |
| e (−) | Fractionation during mitochondrial respiration | –6‰ | Ghashghaie et al. (2003) |
| δ13Cpin (−) | Pinitol δ13C in needle | −30.5‰ | Measurements, see Fig. S2(d) |
| (−) | Equilibrium fractionation during vaporization | – | Majoube (1971) |
| (−) | Fractionation during diffusion of water vapor through boundary layer | 19‰ | Merlivat (1978) |
| (−) | Fractionation during diffusion of water vapor through stomata | 28‰ | Merlivat (1978) |
| f 1 (−) b | Ratio of enriched to total needle water | 0.93 | Calibrated |
| L (m) c | Leaf mesophyll effective mixing length | 0.03 | Calibrated |
| W (mol m−2) | Leaf mesophyll water volume | 5.6 | Measurements, see Fig. S6 |
| (−) | Biochemical fractionation factor | 27‰ | Sternberg et al. (1986) fig. 1 in Sternberg & Ellsworth (2011) |
| δ18Opin (−) | Pinitol δ18O in needle | 25‰ | Calibrated |
| S sug (μmol of C m−2) | Concentration of needle sugar | 1.96 × 105 | Measurements, see Fig. S5 |
| S pin (μmol of C m−2) | Concentration of needle bulk sugars | 0.7 S sug | Measurements, see Fig. S5(b) |
Parameter units are given in parenthesis, where ‘–’ stand for unitless and all area‐based units refer to all‐sided leaf area.
Two‐pool approach.
Péclet approach.
Average mesophyll water (R lw,ss) is less 18O‐enriched compared to the sites of evaporation, which at steady state is commonly formulated as a reduction factor (f 1) above source water:
| (Eqn 4) |
There are two alternative concepts for defining f 1: (1) the two‐pool model, where f 1 is a constant (Leaney et al., 1985); and (2) the Péclet model, which relates f 1 to transpiration (Farquhar & Lloyd, 1993). In the latter , where ; L (in meters) is the effective mixing length, E (in mol m−2 s−1) the transpiration rate, C (55.5 × 103 mol m−3) the molar density of liquid water, and D (2.66 × 10−9 m2 s−1) the diffusivity of H2 18O in liquid water.
At sub‐daily timescales and at times of low transpiration, the steady‐state assumption may be violated because of the slow turn over rate of leaf water (Farquhar & Cernusak, 2005; Gessler et al., 2013). The formulation for the isotopic ratio of oxygen in leaf water under nonsteady state (R lw) is given by (Farquhar & Cernusak, 2005)
| (Eqn 5) |
where W (in mol m−2) is the leaf water content.
New assimilates are generally assumed to be in oxygen isotopic equilibrium with bulk leaf water (Barbour et al., 2000): , where α wc (= 1 + ε wc) is the biochemical fractionation associated with oxygen isotope exchange between carbonyl oxygen and water (Sternberg et al., 1986; Sternberg & Ellsworth, 2011). The signal of new assimilates is then carried to the needle sugar pool following Eqn 6, assuming that the needle sugar pool is well‐mixed and forms the substrate for mitochondrial respiration (Wingate et al., 2007; Ogée et al., 2009).
| (Eqn 6) |
where R sug is the isotopic ratio in the needle sugar pool, S sug (in μmol of C m−2) the concentration of needle sugar, A n net CO2 exchange, r d mitochondrial respiration, and q the discharge of sugars from the needle into the phloem (all in μmol m−2 s−1). Finally, the isotopic ratio of needle WSC is computed as
| (Eqn 7) |
where R pin and S pin (in μmol of C m−2) are the isotopic ratio and concentration of pinitol in the needle, respectively.
Formation of needle sugar δ13C
The model for 13C‐discrimination of net CO2 exchange (, Eqn 8) was adopted from Wingate et al. (2007) (see also Methods S2). They modify the classical equation by Farquhar et al. (1982) to account for the fact that the δ13C composition of the substrate used for mitochondrial respiration may differ from that of current assimilates. Here we assume that substrate is the needle sugar pool. Unlike the formulation in Farquhar et al. (1982) that requires photosynthesis to model the δ13C of the respiratory substrates, this formulation is also valid in absence of photosynthesis (Wingate et al., 2007); during nighttime (k = 0), it simply reduces to the 13C‐discrimination of dark respiration.
| (Eqn 8) |
where C a, C s, C i and C c (in μmol mol−1) are CO2 mole fractions in the atmosphere, at the leaf surface, in the intercellular spaces and in the chloroplasts; R a and R sug are isotopic ratios of CO2 in ambient air and in the sugar pool; a b, a s, a m, b, f and e are fractionation factors associated with diffusion through the boundary layer, diffusion through stomata, transfer through mesophyll, carboxylation, photorespiration, and mitochondrial respiration, respectively (Table 1); Γ * (in μmol mol−1) is the CO2 compensation point in the absence of mitochondrial respiration (Bernacchi et al., 2001) and; k (= (A n + r d)/(C c – Γ *)) is the carboxylation efficiency. Eqn 8 ignores ternary effects as they are also ignored in modeling shoot gas exchange (Methods S1). Such approach is common for mechanistic modeling studies simulating both gas exchange and 13C‐discrimination (Ogée et al., 2009; Schiestl‐Aalto et al., 2021). According to Farquhar & Cernusak (2012) ignoring or accounting for ternary effects consistently in both gas exchange and discrimination calculations lead to almost equivalent results, whereas their inconsistent use in either gas exchange or discrimination calculations produce misleading results. Eqn 8 further assumes there is effectively only one carbon pool, where carbon compounds left behind by respiratory processes mix with the pool of respiratory substrate and carbon in the Calvin–Benson–Bassham (CBB) cycle. Busch et al. (2020) recently suggested an alternative model where respiration is isotopically disconnected and metabolites are not fed back into the CBB cycle, but this possibility was not included in our analysis.
The isotopic ratio of net CO2 exchange is R a/(1 + 13Δ), thus the isotopic signal of carbon in the needle sugar pool is solved from:
| (Eqn 9) |
The δ13C of the needle WSC pool is computed as for oxygen (Eqn 7). The right‐hand side of Eqn 9 is equivalent to .
Materials and Methods
Study site and measurements
Site description
The study site is the Station for Measuring Forest Ecosystem‐Atmosphere Relations (SMEAR II) in Hyytiälä, southern Finland (61°51′N, 24°17′E; Hari & Kulmala, 2005). The site is a managed boreal forest on a shallow mineral soil, with an overstorey dominated by 60‐yr‐old Scots pines (Pinus sylvestris L.). The long‐term (1981–2010) mean annual temperature and precipitation is 3.5°C and 711 mm, respectively (Pirinen et al., 2012). Snow typically covers the ground from December to April.
Shoot gas exchange
Shoot gas exchange measurements were performed with an automated chamber system (Aalto et al., 2014) consisting of a chamber, sample tubing and a gas analyzer. The box shaped shoot chamber (2.1 dm3) made of acrylic plastic and inner surfaces coated with fluorinated ethylene propylene (FEP) film was installed in the uppermost canopy. The chamber enclosed a 1‐yr‐old (in 2018) shoot in horizontal position. The inserted shoot was debudded before the chamber installation to prevent new growth. The chamber was open most of the time exposing the shoots to ambient conditions and closed intermittently for 1 min 50–90 times d–1. Sample air was drawn from the chamber along polytetrafluoroethylene tubes (internal diameter 4 mm, length 73 m) to a gas analyzer (LI‐840; Li‐Cor, Lincoln, NE, USA) and compensated by ambient air leaking freely into the chamber. Fluxes of CO2 and water were determined by fitting a nonlinear equation to concentration records during the first 5–35 s of chamber closure (Kolari et al., 2012). Water fluxes were rejected when RH was > 85% due to considerable adsorption of water on the chamber walls. Fluxes were calculated for all‐sided needle area (Kolari et al., 2007) and re‐sampled to half‐hourly time series. Based on all‐sided needle area measurements the specific leaf area was 0.010 m2 g−1. Resulting data coverage for fluxes of CO2 and water were 98% and 63%, respectively, for mid‐April to mid‐October in 2018–2019.
Sampling and isotopic analysis of water
Samples of 1‐yr‐old needle (1N) water, twig water, soil water and atmospheric water vapor were collected every 3–5 wk during May–October in 2018 and 2019, except for 1N water sampled more frequently in 2019 (14 times + diurnal course on 23 May). Furthermore, 1N and twig samples were collected from five mature Scots pine trees between midday and 15:00 h (utc + 2) from a sun‐exposed position in canopy top. For each tree, 1N samples and the linked twigs with barks peeled off were collected into separate 12 ml exetainer vials (Labco, Lampeter, UK). Soil samples were cored using a foot‐step soil probe from three spots close to the sampled trees. Subsamples were taken from 2, 10 and 18 cm depths, and placed into individual exetainers. Water vapor was collected at 18 m height by pumping air for 2 to 3 h through a hose into a glass U‐tube, which was immersed in a mixture of dry ice and ethanol. Additionally, monthly precipitation samples were collected from May 2018–December 2019 using an evaporation‐free rainwater collector (Gröning et al., 2012). All collected samples were placed in a cool box with ice blocks immediately in the field and stored at −20°C.
Water vapor and precipitation (May–November 2018) samples were melted and transferred into 2 ml vials and analyzed for δ18O at the Stable Isotope Laboratory of Luke (SILL, Helsinki, Finland) by HT‐EA‐IRMS, that is, high temperature (HT) elemental analyzer (Sercon Ltd, Crewe, UK) connected to isotope ratio mass spectrometry (20–22 IRMS; Sercon). The results were calibrated against two in‐house reference waters. Water from plant and soil samples was cryogenically extracted by vacuum distillation (West et al., 2006). The δ18O of plant and soil extracted water of samples from 2018 were determined at the Stable Isotope Research Laboratory of WSL (Birmensdorf, Switzerland) (Lehmann et al., 2020), while corresponding samples from 2019 and precipitation from December 2018 to December 2019 were analyzed at the Stable Isotope Laboratory of University of Basel, Switzerland. For all samples, measurement precision derived from repeated measurements and a quality control water sample was 0.3‰ or better.
Sampling and isotopic analysis of water‐soluble needle carbohydrates
Current‐year needles (0N) and/or 1N were collected c. 20 times during May to October in 2018 and 2019 (+ diurnal course on 25 July 2019), from five trees during same time and canopy position as for water sampling. Samples were placed in a cool box with ice blocks immediately after collection and microwaved at 600 W for 1 min within 2 h to stop enzymatic and metabolic activities (Wanek et al., 2001). Samples were subsequently dried at 60°C for 24 h and homogenized into a fine powder by a ceramic‐ball mill using FastPrep‐24TM. Extraction and purification of WSC were performed according to Wanek et al. (2001) and Rinne et al. (2012). Shortly, the supernatant from the water extraction at 85°C was separated and purified using three types of sample treatment cartridges, which removed amino acids, organic acids and phenolic compounds. The purified samples were freeze‐dried, dissolved in Milli‐Q water, filtered through a 0.45 μm syringe filter (Acrodisc; Pall Corp., Port Washington, NY, USA) and stored at −20°C.
Bulk isotope analysis of WSC was performed at SILL. Before analysis, aliquots of solubilized extracted WSC were pipetted into individual tin and/or silver capsules (IVA Analysentechnik, Meerbusch, Germany), lyophilized and wrapped. The δ13C values were determined using EA‐IRMS (samples of 2018) or concurrently with δ18O analysis by HT‐EA‐IRMS using the dual isotope method (samples of 2019; Woodley et al., 2012). The EA‐IRMS results for δ13C were calibrated against IAEA‐C7 (−32.15‰), IAEA‐CH3 (−24.72‰) and an in‐house sucrose (−12.22‰; Sigma‐Aldrich) reference materials. The dual isotope measurements (δ18O and δ13C) were calibrated against IAEA‐601 (23.14‰ and −28.81‰), in‐house sucrose (36.62‰ and −12.22‰) and lactose (21.05‰ and −24.66‰) standards (Sigma‐Aldrich). Additionally, a subset of the samples were analyzed for δ13C using both the EA and the HT‐EA method to calibrate the HT‐EA results for δ13C (Woodley et al., 2012). Analytical precision of the measurements was 0.2‰ or better for δ13C and δ18O, determined from repeated measurements of a quality control standard.
Compound‐specific isotope analysis of δ13C was performed for the WSC samples of 2018 only, as analysis of samples of 2019 were delayed due to instrumental problems. CSIA was done at WSL using a Delta V Advantage IRMS (Thermo Fisher Scientific, Waltham, MA, USA) coupled to a high‐performance liquid chromatography (HPLC) system with a Finnigan LC Isolink interface (Thermo Fisher Scientific) (Rinne et al., 2012). The δ13C values were determined for the four detected sugars or sugar‐like compounds: sucrose, glucose, fructose and pinitol. External compound‐matched standard solutions with comparable concentration (20–180 ng C μl−1) and δ13C values, as determined by EA‐IRMS, were analyzed between every 10 samples to correct the CSIA results (Rinne et al., 2012). The measurement precision (standard deviation, SD) of sucrose, glucose, fructose and pinitol standards were 0.44‰, 0.57‰, 0.88‰, and 0.38‰, respectively.
The concentration of each four individual compounds from HPLC‐IRMS were calculated using its peak area, and the linear regression between the carbon content and peak area of the compound‐matched standard (Rinne et al., 2012). The concentration of bulk WSC was calculated from the ratio of the sample weight in the tin/silver capsule to the sample weight used for the hot water extraction.
Based on CSIA, needle sugars comprised mainly of sucrose, while the total contribution of glucose and fructose was on average 25%. The concentration‐weighted average δ13C of the sugars was highly correlated with δ13C of sucrose (r > 0.95; Fig. S1), and hence δ13C of sucrose is used to indicate the δ13C of the total needle sugars hereafter.
The δ18O and δ13C of bulk WSC, and δ13C of sucrose and pinitol were each combined over the two needle generations (0N and 1N) to form time series covering the whole growing season (Fig. S2). The δ18O of bulk WSC and δ13C of sucrose were similar for 0N and 1N and the combined dataset was obtained as their average (Fig. S2a,c). Bulk WSC in 2018 and pinitol showed a difference in level of δ13C between 0N and 1N, which was first corrected for before averaging over the two data series (Fig. S2b,d).
Model runs, parameterization and evaluation
The model was ran for the two growing seasons, 2018–2019, using half‐hourly meteorological data measured above the canopy. Additionally, soil moisture from c. 5 cm depth in mineral soil was used as proxy for water availability limiting photosynthesis during dry conditions (Eqn S10 in Methods S1; Launiainen et al., 2022). Isotopic input data was not measured at the site at the resolution needed for the modeling. The δ18O of water vapor (six‐hourly) was obtained from the isotope‐enabled, nudged atmospheric general circulation model IsoGSM (Yoshimura et al., 2011, 2008), which corresponded reasonably well to the limited number of observations (Fig. S3). The δ18O of source water was modeled at daily resolution based on monthly precipitation δ18O, the amount of precipitation, soil moisture and eddy covariance‐based evapotranspiration, resulting in a good fit with the observed twig water δ18O (see Methods S3; Fig. S4). Lastly, δ13C of atmospheric CO2 was available at weekly resolution from Pallas‐Sammaltunturi GAW‐station (White et al., 2015).
Initially, the model results related to leaf gas exchange (A n, E and C i/C a) were evaluated against shoot chamber measurements. Thereafter, the results of the isotopic model were compared against measured δ18O of leaf water, δ18O and δ13C of needle WSC, and δ13C of needle sugars. The model fit was evaluated using mean absolute error (MAE) and R 2.
The parameters applied in the isotopic modeling are listed in Table 1. The measured WSC concentrations indicated little variability during the growing seasons (Fig. S5a). The concentrations fluctuated c. 95 mg g−1, which corresponds to 3.33 × 105 μmol of C m−2, when using the measured specific leaf area (0.010 m2 g−1) and the molecular mass of carbon in sucrose 28.5 g (mol C)−1 (similar to 27.7 and 30 g (mol C)−1 for pinitol and glucose/fructose, respectively). The ratio of pinitol to total sugar concentration was also rather invariant during the measurement period, especially after the beginning of July (Fig. S5b). The ratio was c. 0.7, meaning that sugars accounted for 59% of WSC and pinitol for 41%. Thus, S sug was defined as a constant (Table 1) and hence the discharge from the sugar pool in Eqns 6 and 9 was set equal to net CO2 exchange (q = A n). Lastly, pinitol δ13C was set to −30.5‰ based on CSIA results (Fig. S2d), whereas pinitol δ18O was calibrated in the absence of CSIA results for oxygen.
For both oxygen and carbon isotopic models, we applied a selection of model variants to understand the role of different processes in capturing intra‐seasonal variation of needle sugar δ13C and δ18O. For oxygen, the model variants for needle water δ18O included the Craig–Gordon model (Eqn 2), the two‐pool model (Eqn 4 with constant f 1) and the Péclet model (Eqn 4 with transpiration‐dependent f 1). Each of these models were run both in steady and nonsteady state. Furthermore, to predict needle WSC δ18O, we tested the model with a constant and a temperature‐dependent ε wc (Table 1); and by defining the size of the sugar pool corresponding to the measurements (Table 1) or as a 20‐fold smaller value. The latter resulted in δ18O of leaf water with an offset of ε wc (or δ13C of net CO2 exchange) to be approximately equal to the isotopic values of the needle sugar pool (i.e. all sugars in needle represent current assimilates).
For carbon, we tested how much neglecting mesophyll resistance affects the model results by assuming C c = C i. The importance of photorespiration and mitochondrial respiration were tested by setting f and r d to zero, respectively. With f = r d = 0, Eqn 8 reduces to its most simple formulation. In addition to b = 29‰, this most simple formulation was ran with b = 27‰ and C c = C i, where the lower b implicitly accounts for all isotopic effects that happen during photosynthetic discrimination including the contribution of mesophyll resistance (Farquhar et al., 1982; Ubierna & Farquhar, 2014). With r d = 0, Eqn 8 reduces to the commonly applied formulation that only accounts for diffusion, carboxylation and photorespitation (e.g. Seibt et al., 2008), whereas setting e = 0 would still consider the release of respired CO2 into intercellular spaces (Wingate et al., 2007). Finally, as for oxygen, the effect of the size of the sugar pool was investigated.
Evaluating formation period and environmental/physiological signals
For model results and observations, we examined the relationships between isotopic signals and environmental/physiological variables, that is, needle sugar δ18O against RH and δ13C against C i/C a. Pearson's correlation coefficient (r) was used to quantify the strength of the linear relationship.
To account for the integration over time in the needle sugar pool, we calculated a weighted mean of past RH and C i/C a based on the same assumptions as implemented in the model (i.e. well‐mixed sugar pool of constant size). The implicit solution of Eqn 6 (or Eqn 9 when neglecting e) defines the sugar pool signal at time t as:
| (Eqn 10) |
where and Δt (in seconds) is the time interval between t − 1 and t. Applying Eqn 10 recursively the sugar pool signal can be written as a weighed mean of past time instances .
| (Eqn 11) |
where τ is the number of time steps to consider (here cut off at ) and w n is the weight of the signal at time t – n expressed as:
| (Eqn 12) |
Eqn 11 was used to calculate a weighted RH and C i/C a by replacing R by RH and C i/C a, respectively. Furthermore, the length of the signal formation period (τΔt) over the two growing seasons was examined. For step‐by‐step derivation of Eqns (Eqn 10), (Eqn 11), (Eqn 12) see Methods S4.
Results
Environmental conditions and shoot gas exchange
The 2018 growing season was hotter and drier than 2019, as indicated by higher temperature and vapor pressure deficit (VPD), and lower top soil moisture (Fig. 1a,b). May–September precipitation was higher in 2018 (345 mm) than in 2019 (293 mm), but it was more evenly distributed in 2019 (not shown). During both years, temperature and VPD reached maximum values in July and PAR followed a bell‐shape reaching highest values in June (Fig. 1a,b). Shoot transpiration and net CO2 uptake had distinct seasonal cycles with highest daily fluxes reached in late June to mid‐July (Fig. 1c,e). Low soil moisture in August–September 2018 (Fig. 1b) limited photosynthesis, as shown by model runs with and without considering the water limitation (black vs gray lines in Fig. 1c,e). The value of C i/C a was also affected by the water limitation (Fig. 1g). Overall, the model reproduced shoot gas exchange well (Fig. 1d,f,h). At half‐hourly timescale, R 2 for transpiration and net CO2 uptake were 0.86 and 0.90, respectively.
Fig. 1.
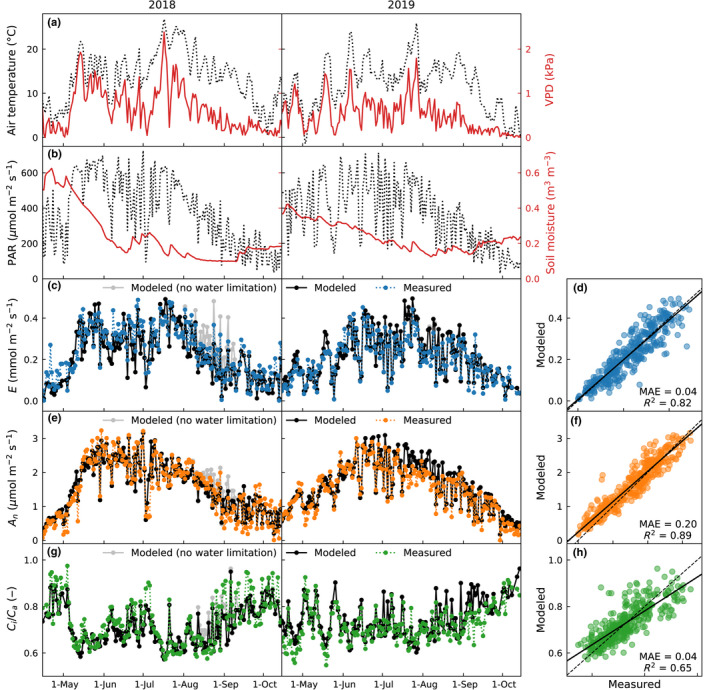
Environmental conditions and Scots pine shoot gas exchange during mid‐April to mid‐October 2018 and 2019. Daily mean (a) air temperature and vapor pressure deficit (VPD), and (b) photosynthetically active radiation (PAR) and soil moisture at 5 cm depth. Modeled and measured (c) transpiration, E, (e) net CO2 uptake, A n, and (g) intercellular to ambient CO2 concentration ratio, C i/C a. E and A n are presented as daily means of times with observations and C i/C a as daily medians for time when sun is above horizon. Model results neglecting the water limitation from soil moisture are shown in gray in (c, e, g). The fit between modeled and measured values is examined in (d, f, h), where the dashed line is 1 : 1 and the solid line the linear least squares regression. R 2 and MAE denote the coefficient of determination and mean absolute error, respectively.
The δ18O of needle water, sugars and water‐soluble carbohydrates
Measured needle water δ18O varied between −10‰ and 15‰ during the study period (Fig. 2a), whereas needle WSC δ18O was much less variable, 23–34‰ (Fig. 2b). Modeled needle water δ18O for the entire studied period (including periods between measurements) showed larger variability than measurements (Fig. 2a) because only a few sampling days occurred during low relative humidity (early July 2018 and late growing season of 2019). However comparing only data from sampling days, the model results (including Péclet effect with nonsteady state and a temperature‐dependent ε wc) well reproduced the seasonal variability in needle water and WSC δ18O (Fig. 2a,b), explaining 95% (Fig. 3f) and 84% (Fig. 3a,d) of their variability, respectively. In order to capture the measured needle WSC δ18O, the δ18O of pinitol was adjusted to 25‰, suggesting 18O‐depletion of pinitol in comparison to sugars. Hence, the variability of needle WSC δ18O was reduced compared to the variability modeled for needle sugar δ18O (Fig. 2b).
Fig. 2.
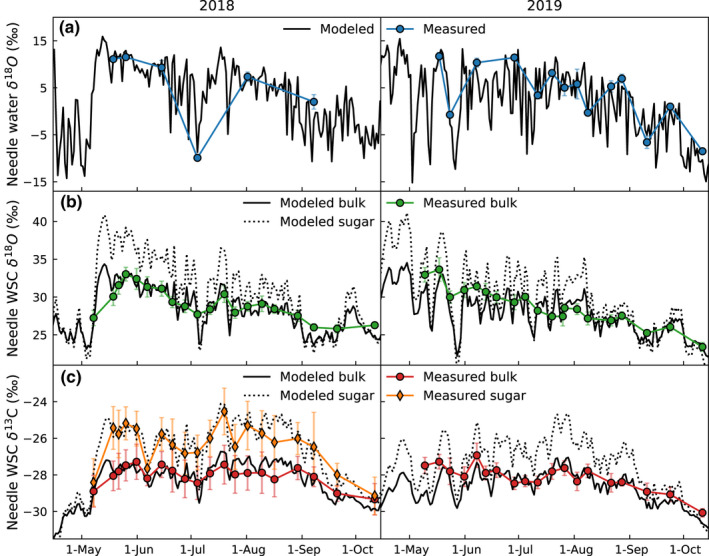
Modeled and measured (a) needle water δ18O, (b) δ18O of needle sugar and in bulk water‐soluble carbohydrates (WSC), and (c) δ13C of needle sugar and in bulk WSC of Scots pine. Modeled values are given as mean of 12:00–15:00 h, which corresponds to sampling interval. Error bars indicate the SDs of the five sampled trees.
Fig. 3.
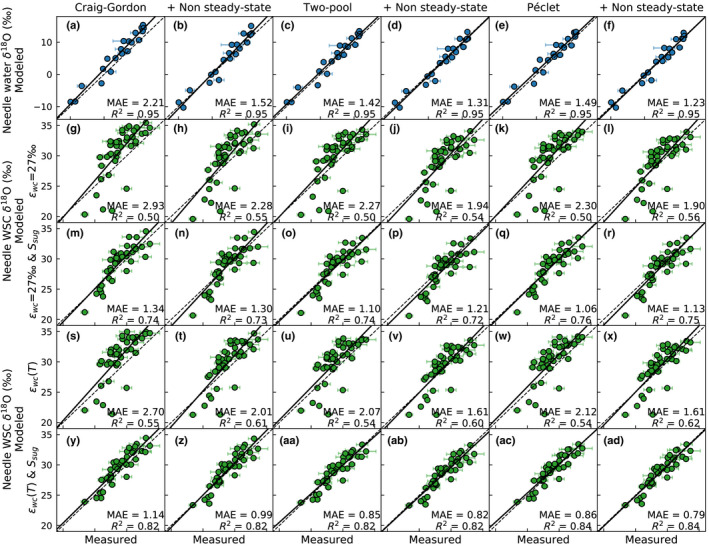
The fit between modeled and measured Scots pine (a–f) needle water δ18O, and (g–ad) δ18O of needle water‐soluble carbohydrates (WSC) with different needle water modeling approaches (see column titles) and model processes affecting δ18O of needle WSC. In (g–r) the biochemical fractionation factor, ε wc, is constant (27‰) and in (s–ad) ε wc is temperature dependent following Sternberg & Ellsworth (2011). In (m–r) and (y–ad) the needle sugar pool size is set according to measurements, while in (g–l) and (s–x) its size is negligible. The dashed line is 1 : 1 and the solid line the linear least squares regression. R 2 and MAE denote the coefficient of determination and mean absolute error, respectively. Modeled values are given as mean of 12:00–15:00 h, which corresponds to sampling interval. Error bars indicate the SDs of the five sampled trees.
The different needle water model variants did not affect the amount of variation captured by the model (R 2 = 0.95, Fig. 3a–f). The level of needle water δ18O was best captured with either the two‐pool or the Péclet model under nonsteady state assumption (MAE = 1.23–1.31‰, Fig. 3d,f), whereas the Craig–Gordon model produced the highest overestimation (Fig. 3a,b). The nonsteady state assumption was also supported by the sampled diel needle water δ18O variation during May 23, 2019 (Fig. S7).
Accounting for the sugar pool size had a significant impact on the fit between modeled and measured δ18O of needle WSC. For example, with constant ε wc, R 2 improved from c. 0.5 (Fig. 3g–l) to 0.72–0.76 (Fig. 3m–r), when the sugar pool size was set according to measurements instead of a negligible size. The model fit improved further when the temperature‐dependent ε wc was implemented, resulting in R 2 ranging from 0.82 to 0.84 (Fig. 3y–ad).
The δ13C of needle sugars and water‐soluble carbohydrates
The temporal variability of observed δ13C of needle bulk WSC was much smaller, with values ranging from −29‰ to −27‰ than that of needle sugar, with values ranging from −29‰ to −24.5‰ (Fig. 2c). This was expected due to the presence of pinitol with a near constant δ13C. The model explained 73% and 60% of the variability in δ13C of needle sugar and bulk WSC, respectively (Fig. 4n).
Fig. 4.
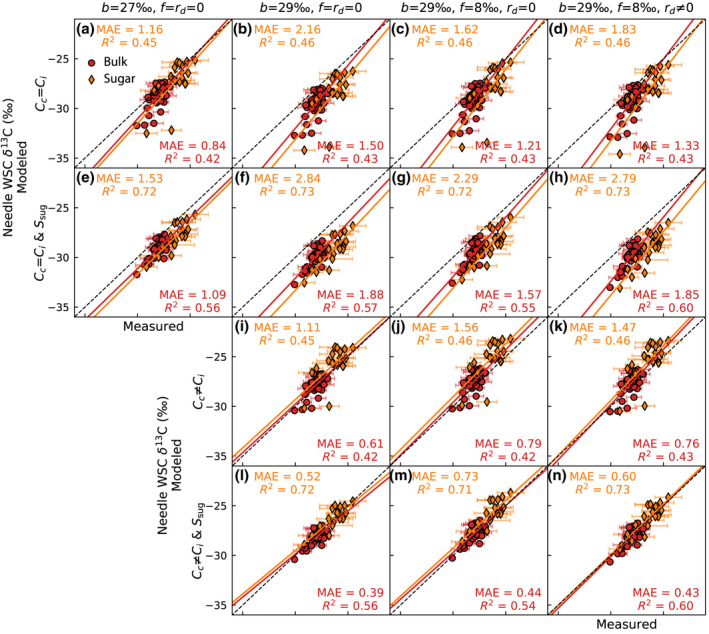
The fit between modeled and measured Scots pine δ13C of needle sugar and in bulk water‐soluble carbohydrates (WSC) with different model variants. The first column shows the commonly applied simple formulation, where the lower b implicitly accounts for all photosynthetic discrimination processes. The second column accounts only for fractionation by diffusion and carboxylation, the third adds photorespiration, and the last the effect of mitochondrial respiration. In (a–h) the models are based on C c = C i and in (i–n) the effect of mesophyll resistance is explicitly accounted for. Lastly, in (e–h) and (l–n) the needle sugar pool size is set according to measurements, while in (a–d) and (i–k) its size is negligible. The dashed line is 1 : 1 and the solid line the linear least squares regression. R 2 and MAE denote the coefficient of determination and mean absolute error, respectively. The upper corner values correspond to sugar and those in lower corner to bulk WSC. Modeled values are given as mean of 12:00–15:00 h, which corresponds to sampling interval. Error bars indicate the SDs of the five sampled trees.
Evaluating the model variants for δ13C of needle sugar and WSC, we observe, as for δ18O, that accounting for the sugar pool size is important. The value of R 2 for needle sugar improved from 0.45–0.46 to 0.71–0.73, when the sugar pool size was accounted for (Fig. 4a–d, i–k vs e–h, l–n, respectively). Applying the model without explicitly accounting for mesophyll resistance captured 72–73% of the variability in observed needle sugar δ13C (Fig. 4e–h). However, in this case, the predicted values were generally too low (Fig. 4d–f) and only the simple model showed a slope close to unity (Fig. 4e). Explicitly accounting for mesophyll resistance (C c ≠ C i) increased the predicted δ13C, which decreased MAE but did not improve R 2 (Fig. 4e–h vs l–n). Similarly, including photorespiration (f = 8‰) had no effect on the variability captured by the model (Fig. 4f,l vs g,m). Then again, including the effect of mitochondrial respiration (r d ≠ 0) slightly improved R 2, especially for δ13C of WSC (Fig. 4g,m vs h,n), which covered two growing seasons, unlike δ13C of needle sugar (Fig. 2c). Overall, the best result (highest R 2 and lowest MAE) was obtained in Fig. 4(n) (b = 29‰, f = 8‰, r d ≠ 0 and C c ≠ C i), which corresponds to the time‐series shown in Fig. 2(c).
Needle sugar pool size and formation period of isotopic signals
As expected, modeling results with a negligible needle sugar pool size showed much larger variability than results obtained applying the observed sugar pool size (Fig. 5a,b). However, during times when day‐to‐day meteorological conditions were fairly stable, e.g. second half of May and early June 2018 (Fig. 1a,b), the role of the sugar pool size was less evident. Then again, the isotopic signal formed during occasional days of low VPD and PAR were not imprinting the sugar pool, because photosynthesis was typically low during these days and hence the role of current assimilates on shaping the isotopic composition of the sugar pool remained small (Fig. 5). The dynamics of the results obtained applying the observed sugar pool size were overall lagged and smoother compared to results with a negligible sugar pool size, especially in early and late growing season when the formation period of the sugar pool isotopic signal was longer (Fig. 5c). During mid‐growing season the sugar pool was formed of photosynthates assimilated over the past 48–52 h, while in early and late growing season the length of the formation period increased to over 5 d (Fig. 5c).
Fig. 5.
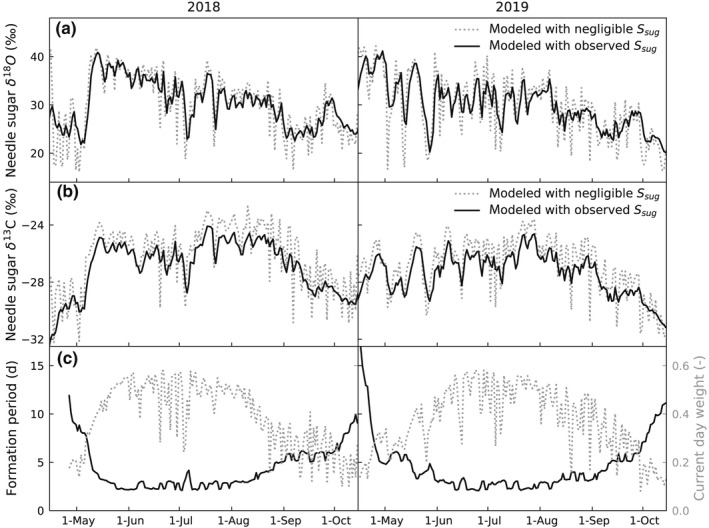
Modeled needle sugar (a) δ18O and (b) δ13C of Scots pine at 13:30 h with negligible sugar pool size and the observed sugar pool size. Panel (c) presents the formation period of the needle sugar pool isotopic signals and the weight of the current day assimilates in forming the isotopic signal of the needle sugar pool. The formation period and the current day weight were calculated assuming the sugar pool is well‐mixed and its size set equal to observed (see text).
The differences between the two modeling scenarios shown in Fig. 5(a,b) are not only caused by the combined effect of the sugar pool and day‐to‐day variation in meteorological conditions, but also the role the sugar pool has on the diurnal course of isotopic signals. Fig. 6 shows the modeled diurnal course of needle water δ18O + ε wc and of δ13C of net CO2 exchange, which during daytime correspond to the sugar pool δ18O and δ13C, respectively, if the sugar pool size were negligible. In comparison to these, the modeled diurnal course of the sugar pool isotopic signals (applying the observed sugar pool size) are lagged and have a much lower amplitude. For δ13C this causes the 1–2‰ offset between the two scenarios in Fig. 5(b) as in the early afternoon δ13C of net CO2 exchange is higher compared to that of the sugar pool (Fig. 6b). For δ18O, the δ18O of needle water + ε wc crosses the value of the sugar pool δ18O around midday (Fig. 6a) and hence Fig. 5(a) does not show a similar offset between the two modeling results as for δ13C. The δ18O and the δ13C of needle WSC measured from diurnal samples during July 25, 2019 support the low diurnal amplitude obtained by the model (Fig. 6a,b).
Fig. 6.
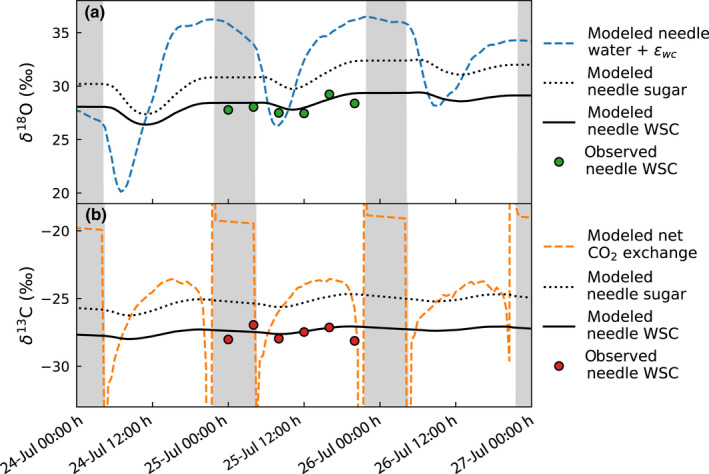
Modeled diurnal course of (a) δ18O and (b) δ13C signals in Scots pine needles during 24–26 July 2019 and measured needle isotopic signals of water‐soluble carbohydrates (WSC) sampled during 25 July 2019. In addition to modeled needle sugar and bulk WSC signals, (a) shows the isotopic signal of needle water + ε wc (biochemical fractionation factor) and (b) the isotopic signal of net CO2 exchange. Gray‐shaded areas indicate night‐time. Extreme high and low values during the transition between day and night are caused by noise in model results when kC a – r d tends to zero (see Eqn 8).
Environmental and physiological signals
The δ18O and relative humidity
Both measured and predicted δ18O of needle water correlated strongly with the sampling day RH (Fig. 7a). The correlation between δ18O of needle WSC and RH was, however, weaker, and deviated from the expected relationship with RH observed for needle water δ18O offset by 27‰ (Fig. 7b). The modeled δ18O of needle sugar deviated less from this relationship but showed a lot of scatter (Fig. 7d). Both for modeled and measured δ18O, the correlations improved considerably, when we used the weighting scheme outlined earlier for RH to account for the sugar pool being an integration over time (Fig. 7b,d vs c,e). Also, accounting for the integration, the relationship between needle sugar δ18O and RH follows the expected line (Fig. 7e).
Fig. 7.
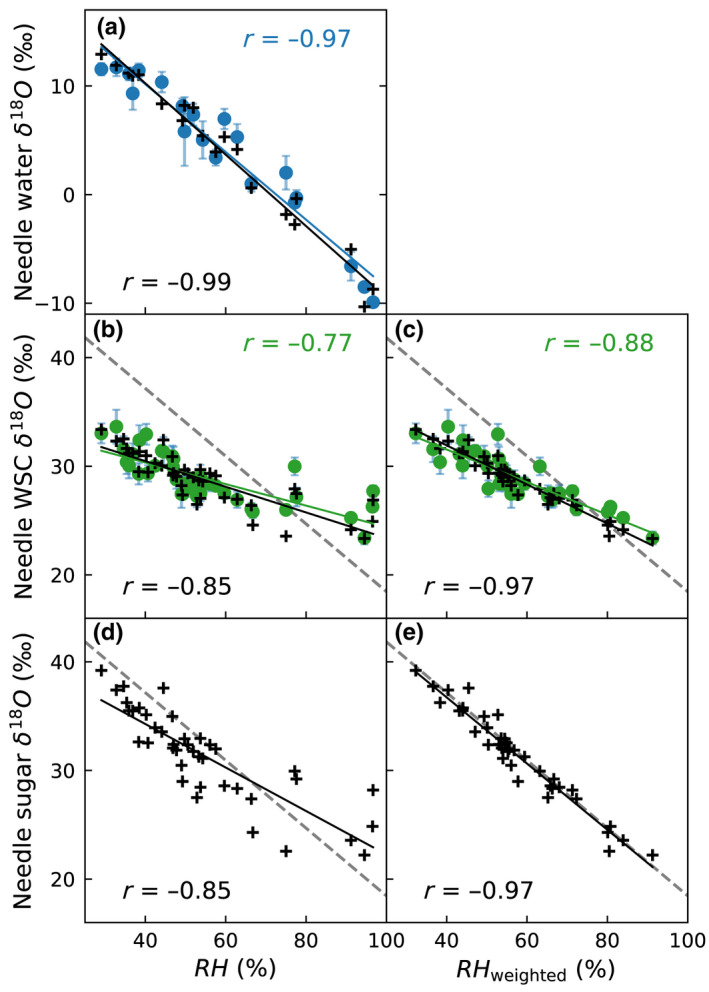
Relationship between relative humidity (RH) and (a) needle water δ18O, (b–c) δ18O of needle water‐soluble carbohydrates (WSC), and (d–e) needle sugar δ18O of Scots pine. In (a, b, d) RH corresponds to the sampling day (mean of midday ± 3 h) and in (c, e) RH is obtained by the weighting scheme outlined in the text. Colored dots and correlations (r) given in upper corner correspond to measurements, and plus‐signs and r given in lower corner to modeled values. The gray dashed line (b–e) indicates the relationship in (a) +27‰. Error bars indicate the SDs of the five sampled trees.
The δ13C and C i/C a
Relationships between δ13C and C i/C a showed a similar response as δ18O to RH; the variability of needle bulk WSC δ13C was reduced compared to that of needle sugar (Fig. 8a,b vs c,d), and the correlation strength increased once time integration was considered (Fig. 8a,c vs b,d).
Fig. 8.
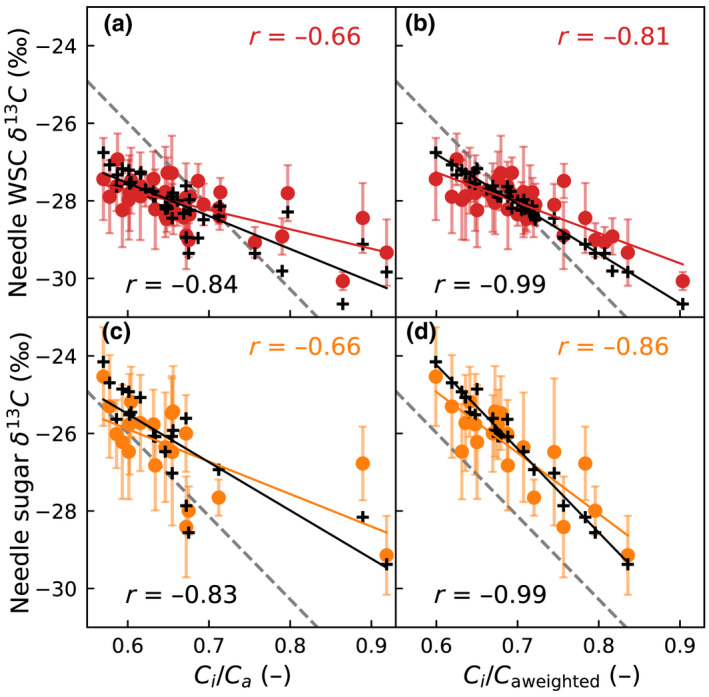
Relationship between modeled intercellular to ambient CO2 concentration ratio (C i/C a) and (a–b) δ13C of needle bulk WSC, and (c–d) needle sugar δ13C of Scots pine. In (a, c) C i/C a corresponds to the sampling day (mean of midday + 3 h) and in (b, d) C i/C a is obtained by the weighting scheme outlined in the text. Colored dots and correlations (r) given in upper corner correspond to measurements, and plus‐signs and r given in lower corner to modeled values. The gray dashed line corresponds to the simple model: 13Δ = a s + (b – a s)C i/C a, with b = 27‰, a s = 4.4‰ and δ13C of atmospheric CO2 set to its mean value −8.5‰. Error bars indicate the SDs of the five sampled trees.
The relationships in Fig. 8 are compared against the expected δ13C vs C i/C a relationship (dashed gray line) defined following the simple model: 13Δ = a s + (b – a s)C i/C a, with b = 27‰ and δ13C of atmospheric CO2 set to its mean value −8.5‰. The slope of this relationship is well represented by the observed and modeled needle sugar δ13C in Fig. 8(d), whereas Fig. 8(a–c) deviate from it because of neglecting the effect of time integration and/or because of the presence of pinitol. The miss‐match in level of c. 1.5‰ in Fig. 8(d) is in line with Fig. 4(e).
Discussion
Evidence for temperature‐dependent biochemical fractionation factor
Modeling of needle water δ18O indicated that its variation was strongly dictated by RH, as both RH (Fig. 7a) and the applied models (Fig. 3a–f) explained c. 95% of the variation of needle water δ18O (Roden & Ehleringer, 1999). Hereby at our study site, the variation in δ18O of water vapor and source water had only minor role, which supports the reconstruction of RH from tree ring δ18O records (Anderson et al., 1998; Wright & Leavitt, 2006). For water vapor δ18O we used IsoGSM data (Yoshimura et al., 2008), a good proxy at our study site (Fig. S3), thus not assuming vapor‐source water isotopic equilibrium (R v = R s/α +, Ogée et al., 2009). Leaf water δ18O is known to be rather insensitive to water vapor δ18O, because the 18O kinetic fractionation dominates over vapor‐source water isotopic disequilibrium (Cernusak et al., 2016). Then again, the minor role of source water δ18O was caused by its limited variability compared to the variability of evaporative 18O‐enrichment (Belmecheri et al., 2018). However, this does not necessarily hold, e.g. at sites where plant water sources vary between isotopically distinct precipitation and melting permafrost (Saurer et al., 2016).
In line with earlier studies, we found that the Craig–Gordon model overestimated needle water δ18O (Cernusak et al., 2016). We could not distinguish whether the two‐pool or the Péclet model provided a more suitable correction as, when calibrated, both models resulted in more or less equally good results (Fig. 3c–f). This has been typical also in earlier studies, but in some cases calibration has led to unrealistic parameter values (Song et al., 2013; Roden et al., 2015). The parameters obtained by calibration here, f 1 = 0.93 and L = 30 mm, are reasonable in comparison to measured leaf anatomical characteristics (Roden et al., 2015; Timofeeva et al., 2020). However, there exist uncertainties related to the calibrated parameter values due to, e.g. the assumption of leaf temperature being equal to air temperature (Ogée et al., 2009; Cernusak et al., 2016) and the source of diffusional fractionation factors (Merlivat, 1978; Cappa et al., 2003).
Based on the diurnal course of needle water δ18O (Fig. S7), we recognized the need for the nonsteady‐state model (Barnard et al., 2007; Gessler et al., 2013). However, its impact on δ18O of needle sugar was less critical (Fig. 3ac vs ad), because during times of highest assimilation δ18O of needle water was close to the steady‐state solution. This suggests steady‐state leaf water models could be sufficient in predicting plant cellulose δ18O (Ogée et al., 2009; Hirl et al., 2021), simplifying model structure and parametrization needs.
One of our key findings was that implementing the temperature‐dependent biological fractionation factor (ε wc), following results from laboratory experiments by Sternberg & Ellsworth (2011), improved predictions of needle WSC δ18O notably (Fig. 3m–r vs y–ad). So far only one study (Hirl et al., 2021) has shown such evidence in field conditions and our study is the first to show this for trees. For our study site, with May–September temperatures varying between −2.9 and 30.7°C, ε wc ranged from 34.6 to 25.4‰. Although this has important implications to predicting plant δ18O signals, here the implemented temperature dependence did not clearly interfere with the dominant effect of RH in driving needle sugar δ18O variability (Fig. 7e). The influence of the temperature‐dependent ε wc is however expected to be critical in climate and leaf temperature reconstruction studies spanning across various temperature zones. For example, as shown by Sternberg & Ellsworth (2011), the temperature‐dependent ε wc may explain the unexpected strong correlation between mean annual temperature and cellulose 18O‐enrichment found across 39 tree species at 25 sites by Helliker & Richter (2008).
A challenge of this study was that δ18O was not measured for needle sugars, but only for bulk WSC. We assumed that WSC consisted of sugars reflecting needle water δ18O and pinitol with a constant δ18O. To capture the observed WSC δ18O, pinitol δ18O was adjusted to 25‰, which is reasonable compared to the 22‰ reported for pinitol in Siberian larch (Lehmann et al., 2017). However, Lehmann et al. (2017) also found differences between δ18O of sucrose (most 18O‐enriched), fructose and glucose, thus there remains some uncertainty in whether it is only the relatively high amounts of 18O‐depleted pinitol that causes the reduced variation in δ18O of needle WSC. While the modeling suggested 18O‐depleted pinitol as one plausible explanation, further compound specific δ18O analyses are needed to verify our finding.
Mesophyll resistance has important role in determining the level of needle sugar δ13C
The C i/C a was the dominant driver of δ13C variation in the modeled needle carbohydrate pools as expected (Farquhar et al., 1982). Increasing model complexity provided only limited improvement to the explained variation. Interestingly, we found that the simple model with the bulk fractionation factor b = 27‰ (Fig. 4e; Farquhar et al., 1982; Ubierna & Farquhar, 2014) captured the variability almost as well as the comprehensive model (Fig. 4n) and only showed a fairly constant offset of 1.5‰. This indicates that changes in C i/C a can be derived from changes in needle sugar δ13C using the simple model (13Δ = 4.4 + (27 – 4.4)C i/C a). However, explicitly accounting for mesophyll resistance (i.e. C c ≠ C i), was required to capture the absolute level of observed δ13C (Warren et al., 2003; Ubierna & Marshall, 2011; Gentsch et al., 2014). The chosen description for mesophyll conductance (Eqn S9 in Methods S1) also played a key role as it defines C c ≈ 0.8C i (Fig. S8a). By contrast, applying a constant mesophyll conductance (e.g. Wingate et al., 2007; Ogée et al., 2009) or a constant ratio between stomatal and mesophyll conductance (e.g. Vernay et al., 2020) result in C c approaching C i at high C i (Fig. S8b,c), which would have led to poorer model agreement, as there was a miss‐match in δ13C values along the whole range of C i/C a (Fig. 8d). This underlines the need for better understanding on the variability of mesophyll conductance at intra‐seasonal scale.
Need to account for needle sugar pool size to predict its isotopic composition
For both δ18O and δ13C, results indicated clearly that the needle sugar pool size has a crucial role in reducing its day‐to‐day (Fig. 5a,b) and sub‐daily (Fig. 6) variation of isotopic composition compared to that of new assimilates. In line with the 13C‐pulse‐labeling study on Pinus pinaster by Desalme et al. (2017), we suggest that the signal of the sugar pool was composed of sugars assimilated over the past 48 h to more than 5 d depending mostly on the time of the growing season (Fig. 5c). While time lags and attenuated diurnal patterns between, e.g. leaf water and leaf organic matter δ18O are commonly recognized (Barnard et al., 2007; Gessler et al., 2013), their causes have not been quantitatively attributed to the sugar pool size, as done here using dynamic modeling. Examining our results at different timescales provides vital information for the interpretation of leaf‐level isotopic data. For example: (1) the difference between instant leaf water δ18O and leaf sugar δ18O seldom equals ε wc (Figs 5a, 6a; Gessler et al., 2013; Lehmann et al., 2017); (2) online measured δ13C of net CO2 exchange (e.g. Wingate et al., 2007; Schiestl‐Aalto et al., 2021) is expected to be higher than δ13C of needle sugar sampled in early afternoon (Fig. 6b); and (3) sudden day‐to‐day variations in meteorological conditions are not strongly reflected in isotopic signals of the needle sugar pool (Fig. 5a,b). Explicitly attributing such phenomena to the simple accumulation and mixing of new assimilates in the needle sugar pool is highly relevant to avoid miss‐interpreting fractionation processes in leaves.
The applied assumption that needle sugars form one well‐mixed pool of constant size may be debatable. It has been suggested that sucrose appears both in a fast transport pool and a slow transport pool (Brauner et al., 2014; Bögelein et al., 2019), which would reduce the variation in needle sugar δ13C further compared to our predictions. The concept of various transport pools might become critical when predicting isotopic compositions further downstream from leaves (e.g. phloem or tree ring cellulose), but here at leaf‐level its role could not be identified. Also, the concentration of needle sugars is expected to vary diurnally (Liesche et al., 2021) and seasonally with increased levels at the start and end of growing season (Schiestl‐Aalto et al., 2019). With high needle sugar concentrations, typical for trees growing under cold winters (Fig. S5; Kagawa et al., 2006; Rinne et al., 2015), we can however expect the diurnal variation in concentration to be small compared to the absolute concentration value. For the seasonal variation, our data showed no clear pattern (Fig. S5), plausibly indicating that during the active growing season, which is the crucial period for tree ring formation, the assumption of constant sugar pool size is sufficient.
Time integration and sample composition critical for retrieving environmental/physiological signals from needle δ18O and δ13C
The correlations between δ18O and RH, and δ13C and C i/C a further underlined the need to account for the needle sugar pool being an integration over time (Figs 7b–e, 8). The correlations were clearly weaker, when examined against sampling day RH and C i/C a compared to RH and C i/C a weighted according to past assimilation and mixing in the sugar pool. The correlation strength between δ13C and C i/C a did not clearly differ for needle WSC and needle sugars (Fig. 8), which supports our assumption on constant pinitol share and pinitol δ13C. The main difference between needle WSC and needle sugars was the slope of the relationships, which for WSC deviated from the expected relationships due to the presence of depleted pinitol (Figs 7c, 8b). This emphasizes the need to know the sample composition, in order to draw conclusions about the magnitude of underlying environmental/physiological changes causing the variation of the isotopic signals (Stokes et al., 2010; Tarin et al., 2020). If the constant share and isotopic signal of pinitol are known, one can estimate changes in RH or C i/C a from bulk WSC δ18O or δ13C, respectively, as the isotopic signal of needle sugar is a linear function of that of bulk WSC (Eqn 7).
Examining the environmental/physiological signals both for model results and observations provided means to explain the phenomena behind the present relationships or the lack of them (see also Hirl et al., 2021). Such evaluation is valuable and has potential to bridge the gap between empirical studies, focused on the climatic signals of tree ring isotopic records, and mechanistic modeling studies, in order to further advance the interpretation of isotopic signals in trees.
Competing interests
None declared.
Author contributions
KL, SL and KTR‐G designed the study. KTR‐G planned the sampling scheme and led the analysis of isotopic data. YT, PS‐A, ES and KTR‐G conducted the fieldwork, YT conducted laboratory preparation of samples, and ES, MS and AK performed the isotope analysis. PK processed the shoot chamber data. KL analyzed the data with assistance from YT and PK, and built the model and run it with guidance from JO, SL and PS‐A. All authors participated in interpreting the results and writing the manuscript in the lead of KL.
Supporting information
Fig. S1 Measured needle sucrose δ13C against measured needle sugar (sucrose + glucose + fructose) δ13C in current‐year needles and 1‐yr‐old needles.
Fig. S2 Measured isotopic composition of needle water‐soluble carbohydrates in current‐year needles and 1‐yr‐old needles, and a combined data series over the two needle generations.
Fig. S3 Measured δ18O of atmospheric water vapor against corresponding values predicted by IsoGSM.
Fig. S4 Modeled and measured source (twig) water δ18O and observed soil water δ18O.
Fig. S5 Measured concentrations of needle water‐soluble carbohydrates and measured ratio of needle pinitol to needle sugar (sucrose + glucose + fructose) concentrations in current‐year needles and 1‐yr‐old needles.
Fig. S6 Measured water content of 1‐yr‐old needles.
Fig. S7 Modeled and measured diurnal course of needle water δ18O.
Fig. S8 Modeled relationship between CO2 mole fraction in chloroplast and intercellular spaces using different descriptions for mesophyll conductance.
Methods S1 Modeling shoot gas exchange.
Methods S2 Derivation of model for 13C‐discrimination of net CO2 exchange (Eqn 8).
Methods S3 Modeling source water δ18O.
Methods S4 Derivation of Eqns (Eqn 10), (Eqn 11), (Eqn 12).
Table S1 Parameter values applied for shoot gas exchange modeling.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
The authors thank Juho Aalto, Bartosz Adamczyk, Teuvo Hietajärvi, Esko Karvinen, Petri Kilpeläinen, Ari Kinnunen, Salla Kuittinen, Tarmo Kylli, Jukka Kärki, Janne Levula, Haoran Li, Marine Manche, Aino Ovaska, Aino Seppänen and Fana Teferra for help in fieldwork and sample preparation; Manuela Oettli for HPLC‐IRMS δ13C analysis; Kei Yoshimura for sharing the IsoGSM data; Sylvia Englund Michel for the data of δ13C of atmospheric CO2. The authors are grateful to Nerea Ubierna and three anonymous reviewers for constructive comments. The work was supported by the Academy of Finland (nos. 332141, 295319) and the European Research Council (no. 755865). PS‐A acknowledges funding from the Knut and Alice Wallenberg Foundation (no. 2015.0047), and SL from the Academy of Finland (no. 296116).
See also the Commentary on this article by Ubierna et al., 236: 2003–2008.
Data availability
The model code was written in Python and is available at https://github.com/LukeEcomod/LeafIsotopes with an example run corresponding to this study. Environmental and eddy‐covariance data for the study site (SMEAR II Hyytiälä forest) can be obtained from https://smear.avaa.csc.fi/. The isotopic and concentration data are available on request to the corresponding author.
References
- Aalto J, Kolari P, Hari P, Kerminen V‐M, Schiestl‐Aalto P, Aaltonen H, Levula J, Siivola E, Kulmala M, Bäck J. 2014. New foliage growth is a significant, unaccounted source for volatiles in boreal evergreen forests. Biogeosciences 11: 1331–1344. [Google Scholar]
- Anderson WT, Bernasconi SM, McKenzie JA, Saurer M. 1998. Oxygen and carbon isotopic record of climatic variability in tree ring cellulose (Picea abies): an example from Central Switzerland (1913–1995). Journal of Geophysical Research: Atmospheres 103: 31625–31636. [Google Scholar]
- Barbour MM, Schurr U, Henry BK, Wong SC, Farquhar GD. 2000. Variation in the oxygen isotope ratio of phloem sap sucrose from Castor bean. Evidence in support of the Péclet effect. Plant Physiology 123: 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour MM, Song X. 2014. Do tree‐ring stable isotope compositions faithfully record tree carbon/water dynamics? Tree Physiology 34: 792–795. [DOI] [PubMed] [Google Scholar]
- Barnard RL, Salmon Y, Kodama N, Sörgel K, Holst J, Rennenberg H, Gessler A, Buchmann N. 2007. Evaporative enrichment and time lags between δ18O of leaf water and organic pools in a pine stand. Plant, Cell & Environment 30: 539–550. [DOI] [PubMed] [Google Scholar]
- Battipaglia G, Saurer M, Cherubini P, Calfapietra C, McCarthy HR, Norby RJ, Cotrufo MF. 2013. Elevated CO2 increases tree‐level intrinsic water use efficiency: insights from carbon and oxygen isotope analyses in tree rings across three forest FACE sites. New Phytologist 197: 544–554. [DOI] [PubMed] [Google Scholar]
- Belmecheri S, Wright WE, Szejner P, Morino KA, Monson RK. 2018. Carbon and oxygen isotope fractionations in tree rings reveal interactions between cambial phenology and seasonal climate. Plant, Cell & Environment 41: 2758–2772. [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Singsaas EL, Pimentel C Jr, Long SP. 2001. Improved temperature response functions for models of rubisco‐limited photosynthesis. Plant, Cell & Environment 24: 253–259. [Google Scholar]
- Bögelein R, Lehmann MM, Thomas FM. 2019. Differences in carbon isotope leaf‐to‐phloem fractionation and mixing patterns along a vertical gradient in mature European beech and Douglas fir. New Phytologist 222: 1803–1815. [DOI] [PubMed] [Google Scholar]
- Brauner K, Hörmiller I, Nägele T, Heyer AG. 2014. Exaggerated root respiration accounts for growth retardation in a starchless mutant of Arabidopsis thaliana . The Plant Journal 79: 82–91. [DOI] [PubMed] [Google Scholar]
- Busch FA, Holloway‐Phillips M, Stuart‐Williams H, Farquhar GD. 2020. Revisiting carbon isotope discrimination in C3 plants shows respiration rules when photosynthesis is low. Nature Plants 6: 245–258. [DOI] [PubMed] [Google Scholar]
- Cappa CD, Hendricks MB, DePaolo DJ, Cohen RC. 2003. Isotopic fractionation of water during evaporation. Journal of Geophysical Research: Atmospheres 108: 4525. [Google Scholar]
- Cernusak LA, Barbour MM, Arndt SK, Cheesman AW, English NB, Feild TS, Helliker BR, Holloway‐Phillips MM, Holtum JAM, Kahmen A et al. 2016. Stable isotopes in leaf water of terrestrial plants. Plant, Cell & Environment 39: 1087–1102. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Ubierna N, Winter K, Holtum JAM, Marshall JD, Farquhar GD. 2013. Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytologist 200: 950–965. [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Wong SC, Farquhar GD. 2003. Oxygen isotope composition of phloem sap in relation to leaf water in Ricinus communis . Functional Plant Biology 30: 1059–1070. [DOI] [PubMed] [Google Scholar]
- Craig H, Gordon LI. 1965. Deuterium and oxygen 18 variations in the ocean and the marine atmosphere. In: Tongiorgi E, ed. Stable isotopes in oceanographic studies and paleotemperatures, Spoleto. Pisa, Italy: Consiglio Nazionale Delle Ricerche, 9–130. [Google Scholar]
- Desalme D, Priault P, Gérant D, Dannoura M, Maillard P, Plain C, Epron D. 2017. Seasonal variations drive short‐term dynamics and partitioning of recently assimilated carbon in the foliage of adult beech and pine. New Phytologist 213: 140–153. [DOI] [PubMed] [Google Scholar]
- Dongmann G, Nürnberg HW, Förstel H, Wagener K. 1974. On the enrichment of H2 18O in the leaves of transpiring plants. Radiation and Environmental Biophysics 11: 41–52. [DOI] [PubMed] [Google Scholar]
- Eglin T, Francois C, Michelot A, Delpierre N, Damesin C. 2010. Linking intra‐seasonal variations in climate and tree‐ring δ13C: a functional modelling approach. Ecological Modelling 221: 1779–1797. [Google Scholar]
- Farquhar GD. 1983. On the nature of carbon isotope discrimination in C4 species. Functional Plant Biology 10: 205–226. [Google Scholar]
- Farquhar GD, Cernusak LA. 2005. On the isotopic composition of leaf water in the non‐steady state. Functional Plant Biology 32: 293–303. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Cernusak LA. 2012. Ternary effects on the gas exchange of isotopologues of carbon dioxide. Plant, Cell & Environment 35: 1221–1231. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Lloyd J. 1993. Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere. In: Ehleringer JR, Hall AE, Farquhar GD, eds. Stable isotopes and plant carbon‐water relations. San Diego, CA, USA: Academic Press, 47–70. [Google Scholar]
- Farquhar GD, O'Leary MH, Berry JA. 1982. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Functional Plant Biology 9: 121–137. [Google Scholar]
- Flanagan LB, Comstock JP, Ehleringer JR. 1991. Comparison of modeled and observed environmental influences on the stable oxygen and hydrogen isotope composition of leaf water in Phaseolus vulgaris L. Plant Physiology 96: 588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch L, Hammerle A, Sturm P, Ogée J, Wingate L, Siegwolf R, Plüss P, Baur T, Buchmann N, Knohl A. 2014. Carbon isotope discrimination during branch photosynthesis of Fagus sylvatica: a Bayesian modelling approach. Plant, Cell & Environment 37: 1516–1535. [DOI] [PubMed] [Google Scholar]
- Gessler A, Brandes E, Keitel C, Boda S, Kayler ZE, Granier A, Barbour M, Farquhar GD, Treydte K. 2013. The oxygen isotope enrichment of leaf‐exported assimilates – does it always reflect lamina leaf water enrichment? New Phytologist 200: 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessler A, Ferrio JP, Hommel R, Treydte K, Werner RA, Monson RK. 2014. Stable isotopes in tree rings: towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiology 34: 796–818. [DOI] [PubMed] [Google Scholar]
- Ghashghaie J, Badeck F‐W, Lanigan G, Nogués S, Tcherkez G, Deléens E, Cornic G, Griffiths H. 2003. Carbon isotope fractionation during dark respiration and photorespiration in C3 plants. Phytochemistry Reviews 2: 145–161. [Google Scholar]
- Gröning M, Lutz HO, Roller‐Lutz Z, Kralik M, Gourcy L, Pöltenstein L. 2012. A simple rain collector preventing water re‐evaporation dedicated for δ18O and δ2H analysis of cumulative precipitation samples. Journal of Hydrology 448–449: 195–200. [Google Scholar]
- Hari P, Kulmala M. 2005. Station for measuring ecosystem‐atmosphere relations (SMEAR II). Boreal Environment Research 10: 315–322. [Google Scholar]
- Helliker BR, Richter SL. 2008. Subtropical to boreal convergence of tree‐leaf temperatures. Nature 454: 511–514. [DOI] [PubMed] [Google Scholar]
- Hirl RT, Ogée J, Ostler U, Schäufele R, Cabrera JCB, Zhu J, Schleip I, Wingate L, Schnyder H. 2021. Temperature‐sensitive biochemical 18O‐fractionation and humidity‐dependent attenuation factor are needed to predict δ18O of cellulose from leaf water in a grassland ecosystem. New Phytologist 229: 3156–3171. [DOI] [PubMed] [Google Scholar]
- IPCC . 2021. Summary for Policymakers. In: Masson‐Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI et al., eds. Climate Change 2021: the physical science basis. Contribution of Working Group I to the sixth assessment report of the Intergovernmental Panel on Climate Change. Cambridge, UK & New York, NY, USA: Cambridge University Press, 3–32. [Google Scholar]
- Kagawa A, Sugimoto A, Maximov TC. 2006. 13CO2 pulse‐labelling of photoassimilates reveals carbon allocation within and between tree rings. Plant, Cell & Environment 29: 1571–1584. [DOI] [PubMed] [Google Scholar]
- Kim Y, Still CJ, Roberts DA, Goulden ML. 2018. Thermal infrared imaging of conifer leaf temperatures: comparison to thermocouple measurements and assessment of environmental influences. Agricultural and Forest Meteorology 248: 361–371. [Google Scholar]
- Kolari P, Bäck J, Taipale R, Ruuskanen TM, Kajos MK, Rinne J, Kulmala M, Hari P. 2012. Evaluation of accuracy in measurements of VOC emissions with dynamic chamber system. Atmospheric Environment 62: 344–351. [Google Scholar]
- Kolari P, Lappalainen HK, Hänninen H, Hari P. 2007. Relationship between temperature and the seasonal course of photosynthesis in scots pine at northern timberline and in southern boreal zone. Tellus Series B: Chemical and Physical Meteorology 59: 542–552. [Google Scholar]
- Launiainen S, Katul GG, Kolari P, Lindroth A, Lohila A, Aurela M, Varlagin A, Grelle A, Vesala T. 2016. Do the energy fluxes and surface conductance of boreal coniferous forests in Europe scale with leaf area? Global Change Biology 22: 4096–4113. [DOI] [PubMed] [Google Scholar]
- Launiainen S, Katul GG, Leppä K, Kolari P, Aslan T, Grönholm T, Korhonen L, Mammarella I, Vesala T. 2022. Does growing atmospheric CO2 explain increasing carbon sink in a boreal coniferous forest? Global Change Biology 28: 2910–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaney FW, Osmond CB, Allison GB, Ziegler H. 1985. Hydrogen‐isotope composition of leaf water in C3 and C4 plants: its relationship to the hydrogen‐isotope composition of dry matter. Planta 164: 215–220. [DOI] [PubMed] [Google Scholar]
- Lehmann MM, Egli M, Brinkmann N, Werner RA, Saurer M, Kahmen A. 2020. Improving the extraction and purification of leaf and phloem sugars for oxygen isotope analyses. Rapid Communications in Mass Spectrometry 34: e8854. [DOI] [PubMed] [Google Scholar]
- Lehmann MM, Fischer M, Blees J, Zech M, Siegwolf RT, Saurer M. 2016. A novel methylation derivatization method for δ18O analysis of individual carbohydrates by gas chromatography/pyrolysis–isotope ratio mass spectrometry. Rapid Communications in Mass Spectrometry 30: 221–229. [DOI] [PubMed] [Google Scholar]
- Lehmann MM, Gamarra B, Kahmen A, Siegwolf RTW, Saurer M. 2017. Oxygen isotope fractionations across individual leaf carbohydrates in grass and tree species. Plant, Cell & Environment 40: 1658–1670. [DOI] [PubMed] [Google Scholar]
- Liesche J, Vincent C, Han X, Zwieniecki M, Schulz A, Gao C, Bravard R, Marker S, Bohr T. 2021. The mechanism of sugar export from long conifer needles. New Phytologist 230: 1911–1924. [DOI] [PubMed] [Google Scholar]
- Lipavská H, Svobodová H, Albrechtová J. 2000. Annual dynamics of the content of non‐structural saccharides in the context of structural development of vegetative buds of Norway spruce. Journal of Plant Physiology 157: 365–373. [Google Scholar]
- Majoube M. 1971. Fractionnement en oxygène 18 et en deutérium entre l'eau et sa vapeur. Journal de Chimie Physique 68: 1423–1436. [Google Scholar]
- McCarroll D, Loader NJ. 2004. Stable isotopes in tree rings. Quaternary Science Reviews 23: 771–801. [Google Scholar]
- Merlivat L. 1978. Molecular diffusivities of H2 16O, HD16O, and H2 18O in gases. The Journal of Chemical Physics 69: 2864–2871. [Google Scholar]
- Offermann C, Ferrio JP, Holst J, Grote R, Siegwolf R, Kayler Z, Gessler A. 2011. The long way down–are carbon and oxygen isotope signals in the tree ring uncoupled from canopy physiological processes? Tree Physiology 31: 1088–1102. [DOI] [PubMed] [Google Scholar]
- Ogée J, Barbour MM, Wingate L, Bert D, Bosc A, Stievenard M, Lambrot C, Pierre M, Bariac T, Loustau D et al. 2009. A single‐substrate model to interpret intra‐annual stable isotope signals in tree‐ring cellulose. Plant, Cell & Environment 32: 1071–1090. [DOI] [PubMed] [Google Scholar]
- O'Leary MH. 1981. Carbon isotope fractionation in plants. Phytochemistry 20: 553–567. [Google Scholar]
- O'Leary MH. 1984. Measurement of the isotope fractionation associated with diffusion of carbon dioxide in aqueous solution. The Journal of Physical Chemistry 88: 823–825. [Google Scholar]
- Pirinen P, Simola H, Aalto J, Kaukoranta J‐P, Karlsson P, Ruuhela R. 2012. Tilastoja Suomen ilmastosta 1981–2010 (climatological statistics of Finland 1981–2010). Helsinki, Finland: Finnish Meteorological Institute. [Google Scholar]
- Richter A, Wanek W, Werner RA, Ghashghaie J, Jäggi M, Gessler A, Brugnoli E, Hettmann E, Göttlicher SG, Salmon Y. 2009. Preparation of starch and soluble sugars of plant material for the analysis of carbon isotope composition: a comparison of methods. Rapid Communications in Mass Spectrometry 23: 2476–2488. [DOI] [PubMed] [Google Scholar]
- Rinne KT, Saurer M, Kirdyanov AV, Bryukhanova MV, Prokushkin AS, Churakova Sidorova OV, Siegwolf RTW. 2015. Examining the response of needle carbohydrates from Siberian larch trees to climate using compound‐specific δ13C and concentration analyses. Plant, Cell & Environment 38: 2340–2352. [DOI] [PubMed] [Google Scholar]
- Rinne KT, Saurer M, Streit K, Siegwolf RT. 2012. Evaluation of a liquid chromatography method for compound‐specific δ13C analysis of plant carbohydrates in alkaline media. Rapid Communications in Mass Spectrometry 26: 2173–2185. [DOI] [PubMed] [Google Scholar]
- Roden J, Kahmen A, Buchmann N, Siegwolf R. 2015. The enigma of effective path length for 18O enrichment in leaf water of conifers. Plant, Cell & Environment 38: 2551–2565. [DOI] [PubMed] [Google Scholar]
- Roden JS, Ehleringer JR. 1999. Hydrogen and oxygen isotope ratios of tree‐ring cellulose for riparian trees grown long‐term under hydroponically controlled environments. Oecologia 121: 467–477. [DOI] [PubMed] [Google Scholar]
- Roeske CA, O'Leary MH. 1984. Carbon isotope effects on enzyme‐catalyzed carboxylation of ribulose bisphosphate. Biochemistry 23: 6275–6284. [DOI] [PubMed] [Google Scholar]
- Saurer M, Kirdyanov AV, Prokushkin AS, Rinne KT, Siegwolf RT. 2016. The impact of an inverse climate–isotope relationship in soil water on the oxygen‐isotope composition of Larix gmelinii in Siberia. New Phytologist 209: 955–964. [DOI] [PubMed] [Google Scholar]
- Schiestl‐Aalto P, Ryhti K, Mäkelä A, Peltoniemi M, Bäck J, Kulmala L. 2019. Analysis of the NSC storage dynamics in tree organs reveals the allocation to belowground symbionts in the framework of whole tree carbon balance. Frontiers in Forests and Global Change 2: 17. [Google Scholar]
- Schiestl‐Aalto P, Stangl ZR, Tarvainen L, Wallin G, Marshall J, Mäkelä A. 2021. Linking canopy‐scale mesophyll conductance and phloem sugar δ13C using empirical and modelling approaches. New Phytologist 229: 3141–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibt U, Rajabi A, Griffiths H, Berry JA. 2008. Carbon isotopes and water use efficiency: sense and sensitivity. Oecologia 155: 441–454. [DOI] [PubMed] [Google Scholar]
- Snyder PK, Delire C, Foley JA. 2004. Evaluating the influence of different vegetation biomes on the global climate. Climate Dynamics 23: 279–302. [Google Scholar]
- Song X, Barbour MM, Farquhar GD, Vann DR, Helliker BR. 2013. Transpiration rate relates to within‐ and across‐species variations in effective path length in a leaf water model of oxygen isotope enrichment. Plant, Cell & Environment 36: 1338–1351. [DOI] [PubMed] [Google Scholar]
- Stangl ZR, Tarvainen L, Wallin G, Ubierna N, Räntfors M, Marshall JD. 2019. Diurnal variation in mesophyll conductance and its influence on modelled water‐use efficiency in a mature boreal Pinus sylvestris stand. Photosynthesis Research 141: 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg L, Ellsworth PFV. 2011. Divergent biochemical fractionation, not convergent temperature, explains cellulose oxygen isotope enrichment across latitudes. PLoS ONE 6: e28040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg LDSL, Deniro MJ, Savidge RA. 1986. Oxygen isotope exchange between metabolites and water during biochemical reactions leading to cellulose synthesis. Plant Physiology 82: 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes VJ, Morecroft MD, Morison JIL. 2010. Comparison of leaf water use efficiency of oak and sycamore in the canopy over two growing seasons. Trees 24: 297–306. [Google Scholar]
- Streit K, Rinne KT, Hagedorn F, Dawes MA, Saurer M, Hoch G, Werner RA, Buchmann N, Siegwolf RT. 2013. Tracing fresh assimilates through Larix decidua exposed to elevated CO2 and soil warming at the alpine treeline using compound‐specific stable isotope analysis. New Phytologist 197: 838–849. [DOI] [PubMed] [Google Scholar]
- Tarin T, Nolan RH, Medlyn BE, Cleverly J, Eamus D. 2020. Water‐use efficiency in a semi‐arid woodland with high rainfall variability. Global Change Biology 26: 496–508. [DOI] [PubMed] [Google Scholar]
- Timofeeva G, Treydte K, Bugmann H, Salmon Y, Rigling A, Schaub M, Vollenweider P, Siegwolf R, Saurer M. 2020. How does varying water supply affect oxygen isotope variations in needles and tree rings of scots pine? Tree Physiology 40: 1366–1380. [DOI] [PubMed] [Google Scholar]
- Ubierna N, Farquhar GD. 2014. Advances in measurements and models of photosynthetic carbon isotope discrimination in C3 plants. Plant, Cell & Environment 37: 1494–1498. [DOI] [PubMed] [Google Scholar]
- Ubierna N, Marshall JD. 2011. Estimation of canopy average mesophyll conductance using δ13C of phloem contents. Plant, Cell & Environment 34: 1521–1535. [DOI] [PubMed] [Google Scholar]
- Vernay A, Tian X, Chi J, Linder S, Mäkelä A, Oren R, Peichl M, Stangl ZR, Tor‐Ngern P, Marshall JD. 2020. Estimating canopy gross primary production by combining phloem stable isotopes with canopy and mesophyll conductances. Plant, Cell & Environment 43: 2124–2142. [DOI] [PubMed] [Google Scholar]
- Wanek W, Heintel S, Richter A. 2001. Preparation of starch and other carbon fractions from higher plant leaves for stable carbon isotope analysis. Rapid Communications in Mass Spectrometry 15: 1136–1140. [DOI] [PubMed] [Google Scholar]
- Warren CR, Ethier GJ, Livingston NJ, Grant NJ, Turpin DH, Harrison DL, Black TA. 2003. Transfer conductance in second growth Douglas‐fir (Pseudotsuga menziesii (Mirb.)Franco) canopies. Plant, Cell & Environment 26: 1215–1227. [Google Scholar]
- West AG, Patrickson SJ, Ehleringer JR. 2006. Water extraction times for plant and soil materials used in stable isotope analysis. Rapid Communications in Mass Spectrometry 20: 1317–1321. [DOI] [PubMed] [Google Scholar]
- White, J , Vaughn, B , Michel, S . 2015. University of Colorado, Institute of Arctic and Alpine Research (INSTAAR), stable isotopic composition of atmospheric carbon dioxide ( 13 C and 18 O) from the NOAA ESRL carbon cycle cooperative global air sampling network, 1990–2014, v.20210204 [www document] URL ftp://aftp.cmdl.noaa.gov/data/trace_gases/co2c13/flask/ [accessed 1 September 2021].
- Wingate L, Seibt U, Moncrieff JB, Jarvis PG, Lloyd J. 2007. Variations in 13C discrimination during CO2 exchange by Picea sitchensis branches in the field. Plant, Cell & Environment 30: 600–616. [DOI] [PubMed] [Google Scholar]
- Woodley EJ, Loader NJ, McCarroll D, Young GHF, Robertson I, Heaton THE, Gagen MH, Warham JO. 2012. High‐temperature pyrolysis/gas chromatography/isotope ratio mass spectrometry: simultaneous measurement of the stable isotopes of oxygen and carbon in cellulose. Rapid Communications in Mass Spectrometry 26: 109–114. [DOI] [PubMed] [Google Scholar]
- Wright WE, Leavitt SW. 2006. Boundary layer humidity reconstruction for a semiarid location from tree ring cellulose δ18O. Journal of Geophysical Research: Atmospheres 111: D18105. [Google Scholar]
- Yoshimura K, Frankenberg C, Lee J, Kanamitsu M, Worden J, Röckmann T. 2011. Comparison of an isotopic atmospheric general circulation model with new quasi‐global satellite measurements of water vapor isotopologues. Journal of Geophysical Research: Atmospheres 116: D19118. [Google Scholar]
- Yoshimura K, Kanamitsu M, Noone D, Oki T. 2008. Historical isotope simulation using Reanalysis atmospheric data. Journal of Geophysical Research: Atmospheres 113: D19108. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Measured needle sucrose δ13C against measured needle sugar (sucrose + glucose + fructose) δ13C in current‐year needles and 1‐yr‐old needles.
Fig. S2 Measured isotopic composition of needle water‐soluble carbohydrates in current‐year needles and 1‐yr‐old needles, and a combined data series over the two needle generations.
Fig. S3 Measured δ18O of atmospheric water vapor against corresponding values predicted by IsoGSM.
Fig. S4 Modeled and measured source (twig) water δ18O and observed soil water δ18O.
Fig. S5 Measured concentrations of needle water‐soluble carbohydrates and measured ratio of needle pinitol to needle sugar (sucrose + glucose + fructose) concentrations in current‐year needles and 1‐yr‐old needles.
Fig. S6 Measured water content of 1‐yr‐old needles.
Fig. S7 Modeled and measured diurnal course of needle water δ18O.
Fig. S8 Modeled relationship between CO2 mole fraction in chloroplast and intercellular spaces using different descriptions for mesophyll conductance.
Methods S1 Modeling shoot gas exchange.
Methods S2 Derivation of model for 13C‐discrimination of net CO2 exchange (Eqn 8).
Methods S3 Modeling source water δ18O.
Methods S4 Derivation of Eqns (Eqn 10), (Eqn 11), (Eqn 12).
Table S1 Parameter values applied for shoot gas exchange modeling.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
The model code was written in Python and is available at https://github.com/LukeEcomod/LeafIsotopes with an example run corresponding to this study. Environmental and eddy‐covariance data for the study site (SMEAR II Hyytiälä forest) can be obtained from https://smear.avaa.csc.fi/. The isotopic and concentration data are available on request to the corresponding author.


