Abstract
Rationale
Inspiratory flow limitation (IFL), characterized by flattening of individual breaths on the airflow/time tracing, is a noninvasive indicator of elevated upper airway resistance. An IFL “event” in isolation has not been defined, nor has the ability to reproducibly identify event occurrence been tested. IFL events and their association with immediate physiological responses—as well as the impact of characteristics such as age, sex, sleep stage, sleepiness, and event duration on their association with such outcomes—have not been studied. Symptomatic patients with a normal to mildly abnormal apnea–hypopnea index who have predominant IFL on their polysomnography may benefit from treatment.
Objectives
To test the reproducibility of identifying IFL events and their termination and to determine the frequency of the immediate physiological response to their occurrence, including desaturation, electroencephalography (EEG) arousal, and increased heart rate (HR).
Methods
Fifty-eight patients with obstructive sleep apnea (OSA) underwent full diagnostic polysomnography. IFL events and their termination were identified manually using predefined rules from the unscored nasal cannula flow channel alone and were evaluated for responses such as EEG arousal, oxygen desaturation of ⩾3%, and HR increase.
Results
Interscorer reliability was acceptable, with an average percent agreement for occurrence of 82% ± 3%. Of all IFL events, 24% (regardless of the definition) were not associated with an EEG arousal, an increase in HR, or O2 desaturation. Of all IFL events scored, 25% caused O2 desaturation, 40% were associated with an EEG arousal, and 55% were associated with an increase in HR; 67% caused either an EEG arousal and/or an increase in HR. Responses were observed to occur either in isolation or in combination. IFL events that terminated with at least two non-IFL breaths, one of which had a 200% increase in amplitude, were significantly associated with O2 desaturation, EEG arousal, and increase in HR compared with events that ended in one non-IFL breath. IFL events that had a >50% reduction in flow amplitude compared with baseline were significantly associated with O2 desaturation compared with events that had a 30% reduction or less.
Conclusions
Most IFL events resulted in immediate physiological responses, and no single consequence reliably occurred after every event. We propose a framework that can incorporate the scoring of IFL events into assessing the diagnosis and severity of OSA and suggest that no single consequence be used to define IFL as a respiratory event. The relationship of IFL events to OSA outcomes remains to be tested.
Keywords: inspiratory flow limitation, obstructive sleep apnea, arousal
The diagnosis and the severity of obstructive sleep apnea (OSA) are measured by the widely used apnea–hypopnea index (AHI), which poorly correlates with a patient’s clinical presentation, outcomes, and response to treatment (1, 2). There is concern that subtler forms of sleep-disordered breathing can be missed if the AHI, as variously defined, is the only criteria used. An example would be patients who have few apneas or hypopneas but demonstrate a predominance of inspiratory flow limitation (IFL). IFL reflects upper airway collapsibility, but its quantification is not incorporated into the count of events in the AHI. Using positive airway pressure to correct IFL in addition to treating apneas and hypopneas can improve daytime functioning in patients, suggesting its impact on clinical outcomes (3).
IFL develops during sleep, when the upper airway collapses and the airflow that results from an inspiratory effort is disproportionate to, and usually reduced from, that produced by a similar respiratory effort during wakefulness. The most commonly used model of this phenomenon is of a Starling resistor, which has the characteristic that resistance of the airway increases because of obstruction from inspiratory-effort–related collapse such that flow becomes independent of effort. Condos and colleagues pointed out that IFL is recognizable from flattening of the inspiratory curve on the typical flow/time tracing used in polysomnography. IFL can also result from other patterns of collapse that cause an increase in respiratory resistance or a progressive decrease of the inspiratory flow as effort increases, i.e., negative effort dependence (4). The gold standard for measuring respiratory effort has been esophageal manometry, and this has been used to demonstrate increasing intrathoracic pressure and, thus effort, during IFL. However, esophageal manometry is invasive and impractical for routine clinical studies. We have shown that the pattern of flow on the flow/time tracing obtained during routine sleep studies can be used (primarily by identifying a plateau on the inspiratory flow signal from a nasal cannula/pressure transducer) to indirectly identify increased upper airway resistance. We and others have shown that identifying this pattern of IFL thus provides information comparable to actually measuring esophageal manometry, consistent with the consensus of identifying IFL at a recent American Thoracic Society (ATS) workshop (5–7).
The relationship between IFL and clinical outcomes has mostly been based on observational studies on upper airway resistance syndrome (UARS), which was initially reported by Guilleminault and colleagues (8). The definition of UARS was originally based on the hypothesis that snoring and respiratory-effort–related arousals result in excessive daytime sleepiness and negatively impact cardiovascular and cognitive health. Patients with UARS with continuous IFL tend to display a disruption of sleep as measured by electroencephalography (EEG) despite having a low AHI, but IFL may also result in repetitive hypopneas (9). Respiratory-effort–related arousals have been associated with excessive sleepiness, hemodynamic changes, and sympathetic activation (10, 11). Observational studies have shown that sleep-disordered breathing in pregnancy is characterized by IFL and mild OSA and its presence is linked to increased risk of gestational hypertension and diabetes (12). Similarly, pediatric patients with sleep-disordered breathing have mild degrees of stable upper airway obstruction/airway limitation that are associated with neurobehavioral impairment (13). In addition to eliminating apneas and hypopneas, treatment of IFL with continuous positive airway pressure (CPAP) may improve neurocognitive outcomes (3). An ATS workshop established an algorithm for visually identifying a breath as having IFL. However, no recommendations were made about defining a multiple breath sequence or “IFL event.” In particular, there has been no standardized definition for the termination of IFL events (7). The clinical consequences (e.g., excessive daytime sleepiness) in patients with predominantly only IFL during sleep have been incompletely studied. To facilitate this goal, we evaluated formalized event definitions for IFL events and examined their immediate physiological responses.
The purpose of the present study was threefold: 1) to test the reproducibility of two proposed definitions for the termination of an IFL event using the ATS IFL algorithm to identify IFL breaths, 2) to determine the relationship of IFL respiratory events to subsequent physiological responses, such as cortical EEG arousals or autonomic arousals as measured by an increase in heart rate (HR) and O2 desaturation, and 3) to study the effect of sleep stage, event duration, subject age, sex, and sleepiness on the relationship between IFL respiratory events and their aforementioned responses.
Methods
Study Subjects
Fifty-eight polysomnograms (PSGs) were taken from a subsample of subjects who participated in a larger parent study relating OSA to daytime functioning (14). Recruitment for the parent study required being older than 18 years old, having complaints of excessive daytime sleepiness and/or snoring, and having a primary diagnosis of OSA and/or UARS. Exclusion criteria were being unable to provide consent, being pregnant, or having a medically unstable condition, myocardial infarction, congestive heart failure, a change in medications during the trial, and a recent or confirmed history of alcohol or recreational drug abuse. All patients and subjects signed informed consent documents; the protocol for the parent study was approved by the New York University Institutional Review Board (IRB), and IRB approval for data analysis was obtained from the Icahn School of Medicine at Mount Sinai. Analysis of data obtained from the parent study was also approved by the Mount Sinai IRB. Demographics and other characteristics are shown in Table 1. Sixteen percent of the subjects identified themselves as Hispanic; the racial distribution was 55% White , 14% African American, 6% Asian, and 21% other.
Table 1.
Demographic data for all subjects (N = 58)
| Mean | Range | |
|---|---|---|
| Sex | 26 F/32 M | |
| Age, yr | 42 | 21–75 |
| BMI, kg/m2 | 28 | 20.3–47.3 |
| AHI4% score | 4.6 | 0–15 |
| AHI3A score | 8.4 | 0–33.6 |
| ESS score | 9.2 | 1–20 |
| TST, h | 7 | 3.8–9.5 |
| Sleep efficiency, % | 82.7 | 50.3–98 |
| NREM/REM, % | 82/18 | 70–92/8–30 |
| Arousal Index | 16 | 0–29 |
| END1/END2, % of all events | 22/78 | 0–63/37–100 |
Definition of abbreviations: AHI3A = hypopnea with 3% drop in oxygen saturation and/or arousal; AHI4% = apnea–hypopnea index, hypopnea with 4% drop in oxygen saturation; BMI = body mass index; END1 = reappearance of one breath without clear flow limitation (typically sinusoidal) with an amplitude at least 200% of the amplitude of the two preceding breaths with inspiratory flow limitation; END2 = reappearance of at least two breaths without clear flow limitation (typically sinusoidal) of which at least one showed an amplitude at least 200% of the amplitude of the two preceding breaths with inspiratory flow limitation; ESS = Epworth Sleepiness Scale; NREM = non–rapid eye movement; REM = rapid eye movement; TST = total sleep time.
Methods
Details of the protocol for the parent study are described in our previous work (15). Briefly, subjects underwent diagnostic nocturnal polysomnography (NPSG) at the New York University Sleep Disorders Center. All studies consisted of full in-laboratory NPSG (Sandman sleep system; Embla Systems Inc.) performed according to American Academy of Sleep Medicine guidelines. Recordings of frontal, central, and occipital EEG, electrooculogram, and submental electromyogram were used to monitor sleep metrics. Leg movements were monitored with an anterior tibialis electromyogram. A unipolar electrocardiogram was used for cardiac monitoring. Oxygen saturation was monitored with a pulse oximeter (Masimo). Chest wall and abdominal movement were monitored with piezoelectric strain gauges. Sleep position was monitored with a multiposition switch. Respiratory airflow was measured using a nasal cannula/pressure transducer system (Protech PTAF2), along with an oral thermistor to detect mouth breathing. Sleep and respiratory event scoring for the NPSG data was performed according to AASM criteria (AASM Scoring Manual, version 2.5, 2018). The apnea–hypopnea index 4% (AHI4%) defines a hypopnea as a peak signal excursion drop of ⩾30% of the preevent baseline using nasal pressure associated with a 4% decrease in oxygen desaturation. AHI3A defines hypopnea as having a similar reduction in amplitude but requires an associated ⩾3% oxygen desaturation or an arousal. Arousals were scored on the basis of AASM criteria (AASM Scoring Manual, version 2.5, 2018).
From this parent study, we selected patients without OSA (AHI4% ⩽5 events/h) to those with mild OSA (AHI4% >5 or ⩽15) who were not taking medications known to affect sleep and who had completed a complete diagnostic NPSG. IFL events were identified from the unscored nasal cannula channel alone, with the initial scorer blinded to the responses of the event. IFL breaths were scored manually using a scoring algorithm established during a previous ATS workshop (7). IFL events (⩾2 breaths with IFL and duration of at least 10 s) were classified as severe (>50% reduction in flow), moderate (30–50% reduction), or mild (0–30% reduction), with a flattening of the flow inspiratory curve. Flow amplitude reduction was calculated based on subtracting the average of the two smallest IFL breaths within the event related to the running 10 breath average amplitude of breaths with normal sinusoidal flow preceding the event.
Two definitions were used for the termination of an IFL event (see Figure 1): END1 = reappearance of one breath without clear flow limitation (typically sinusoidal) with an amplitude at least 200% of the amplitude of the two preceding breaths with IFL. END2 = reappearance of at least two breaths without clear flow limitation (typically sinusoidal) of which at least one showed an amplitude at least 200% of the amplitude of the two preceding breaths with IFL.
Figure 1.
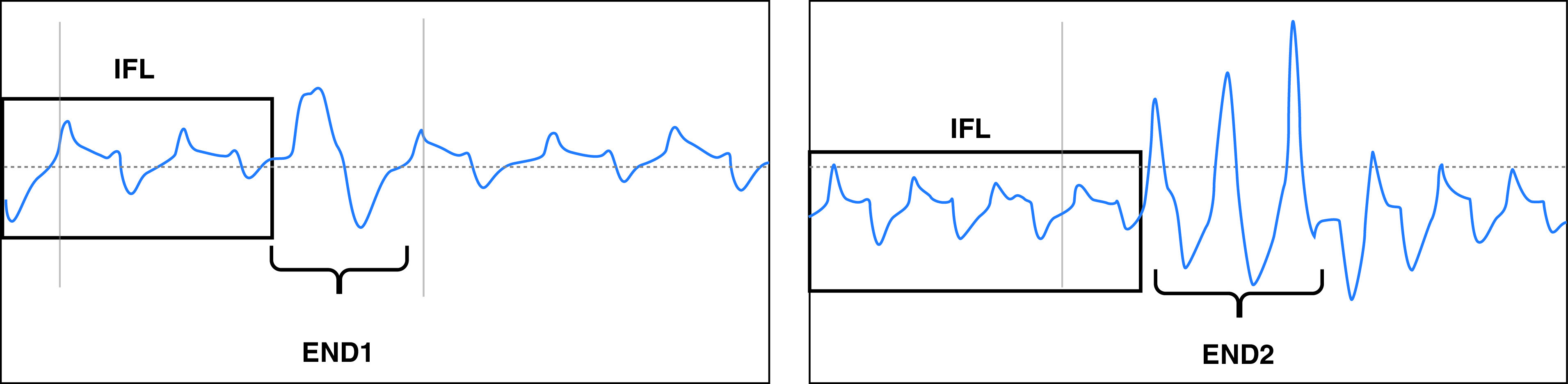
Defining END1 and END2. END1 = reappearance of one breath without clear flow limitation (typically sinusoidal) with an amplitude at least 200% of the amplitude of the two preceding breaths with inspiratory flow limitation; END2 = reappearance of at least two breaths without clear flow limitation (typically sinusoidal) of which at least one showed an amplitude at least 200% of the amplitude of the two preceding breaths with inspiratory flow limitation; IFL = inspiratory flow limitation.
Each respiratory event termination was then linked by custom software to the presence of the following characteristics:
-
1.
Oxygen desaturation ⩾3% within 30 seconds of event termination.
-
2.
EEG arousal as defined by the AASM (linked if they occur within 3 seconds prior to the end of the event or within 5 seconds after the event termination).
-
3.
Increase in HR by 6 peats per minute in the 10 seconds following the event, using as a baseline the average HR during the 5 seconds prior to event termination. Our previous work demonstrated that an increase in HR of 6 bpm for two consecutive beats is significant as a consequence of a respiratory event (16).
Whereas some of the events outlined would be consistent with an AASM hypopnea (i.e., flow limitation >30% with 3% O2 desaturation or arousal), our approach is distinctly different. We prioritize the presence of IFL to define our event and also propose two different definitions for the termination of an event. We also look at events that have a minimal flow reduction (0–30%) but have flattening of the inspiratory curve consistent with IFL, and we also evaluate HR as a significant immediate physiological response to the event.
The distribution of responses related to IFL termination was also evaluated separately in the following categories.
-
1.
Sleep phase (rapid eye movement [REM] and non–rapid eye movement [NREM] sleep)
-
2.
Subject sex (male and female)
-
3.
The duration of the IFL event (10–30 s, >30–120 s, >120 s)
-
4.
Subject age (⩽45 y, >45 y)
-
5.
Sleepiness as measured by the Epworth Sleepiness Scale (⩾10 = sleepy).
Interscorer Agreement for IFL Termination
Four scorers independently identified IFL termination events using the nasal cannula channel alone in five subjects. We chose three normal subjects and two subjects with mild OSA for this exercise. The percent agreement in identifying the presence or absence of an event was calculated across four scorers.
Statistical Analysis
The frequency of occurrence of each consequence for each IFL termination event type was tabulated. The impact of the duration of IFL, age, sex, and sleep stage on the presence of responses associated with IFL termination was also assessed. We used a generalized linear mixed-effects model to incorporate the random effects associated with individual patients to calculate the relationship between our termination definitions (END1 and END2) and immediate physiological responses (O2 desaturation, EEG arousal, and HR change). We derived confidence intervals (CIs) from that model. We used logistic regression model to see whether there was an effect of age, body mass index (BMI), sex, degree of sleepiness (ESS score), duration of IFL event, and sleep stage on the relationship between events and responses.
Results
Interscorer Agreement
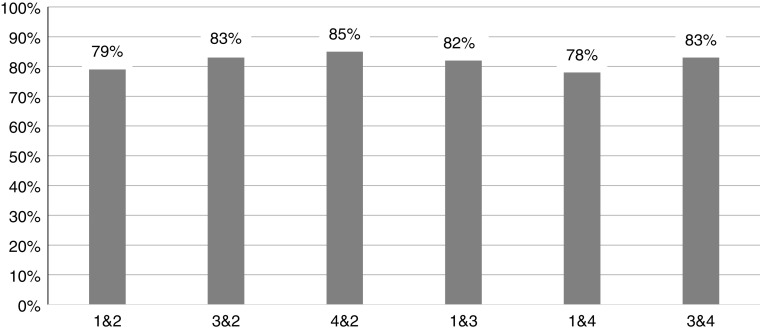
The percent agreement for identifying the presence or absence of an event across four scorers for 548 events is displayed in Figure 2. The average percent agreement was 82 ± 3%.
Figure 2.
Percent agreement among scorers. Four scorers independently identified inspiratory flow limited termination events using the nasal cannula channel alone in five subjects. The average percent agreement among four scorers for the presence or absence of events was 82% ± 3%.
Comparison of physiological responses to events
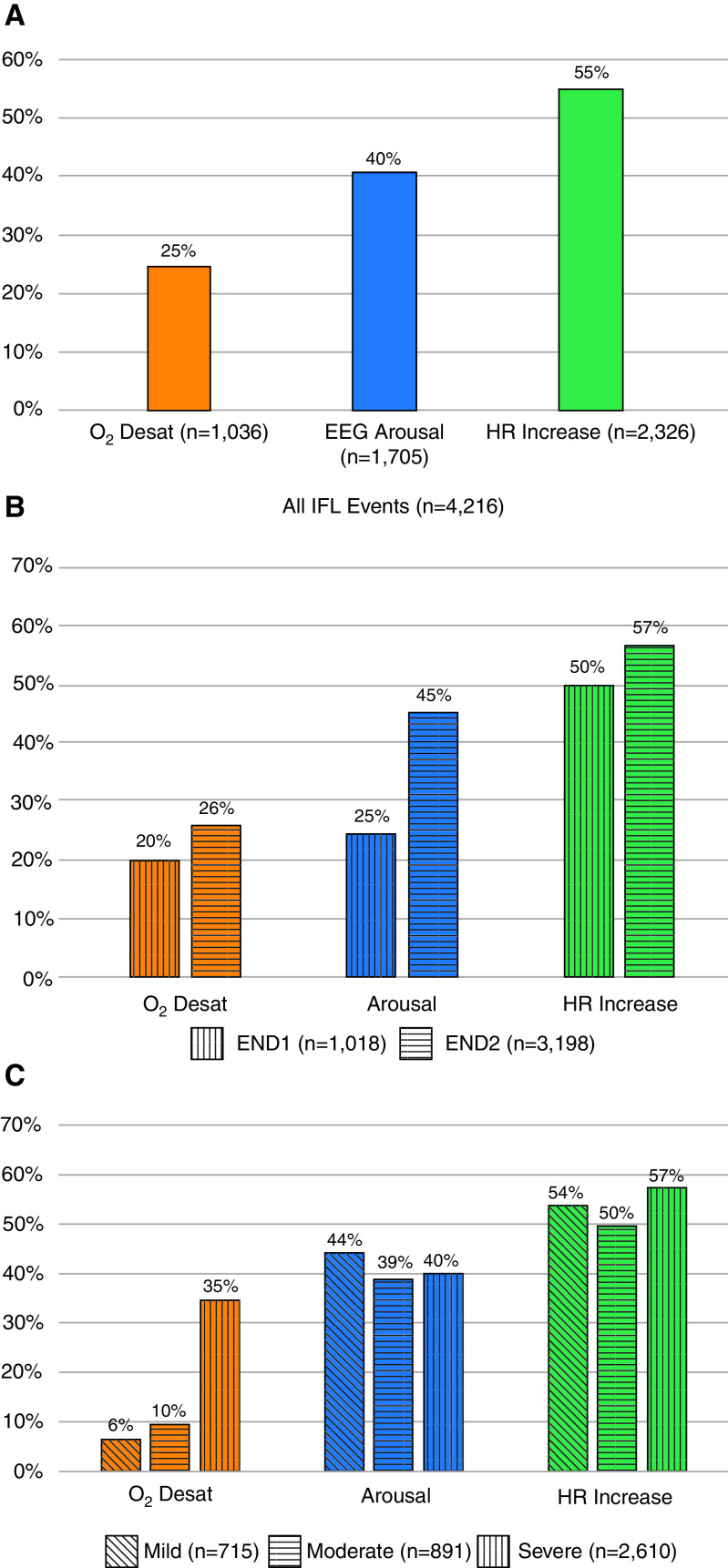
A total of 4,216 IFL events were found in the 58 patients, with an average of 73 events per patient. The most common consequence was increase in HR, which occurred after 55% of the events overall. O2 desaturation (3%) occurred the least, in 25% of the events overall (Figure 3A). The frequency with which the possible combinations of desaturation, EEG arousal, and increase in HR in all IFL events is displayed with a Venn diagram (Figure 4A). IFL events that were associated with O2 desaturation were not necessarily associated with EEG arousal or increase in HR. Similarly, EEG arousals were not always associated with an increase in HR. Only 7% (313) of IFL events were associated with all three responses, and 24% of IFL events showed none of the responses.
Figure 3.
Distribution of immediate physiological responses in (A) all IFL events, (B) by END type, and (C) by degree of flow amplitude reduction. Desat = desaturation; EEG = electroencephalography; END1 = reappearance of one breath without clear flow limitation (typically sinusoidal) with an amplitude at least 200% of the amplitude of the two preceding breaths with inspiratory flow limitation; END2 = reappearance of at least two breaths without clear flow limitation (typically sinusoidal) of which at least one showed an amplitude at least 200% of the amplitude of the two preceding breaths with inspiratory flow limitation; HR = heart rate; IFL = inspiratory flow limitation.
Figure 4.
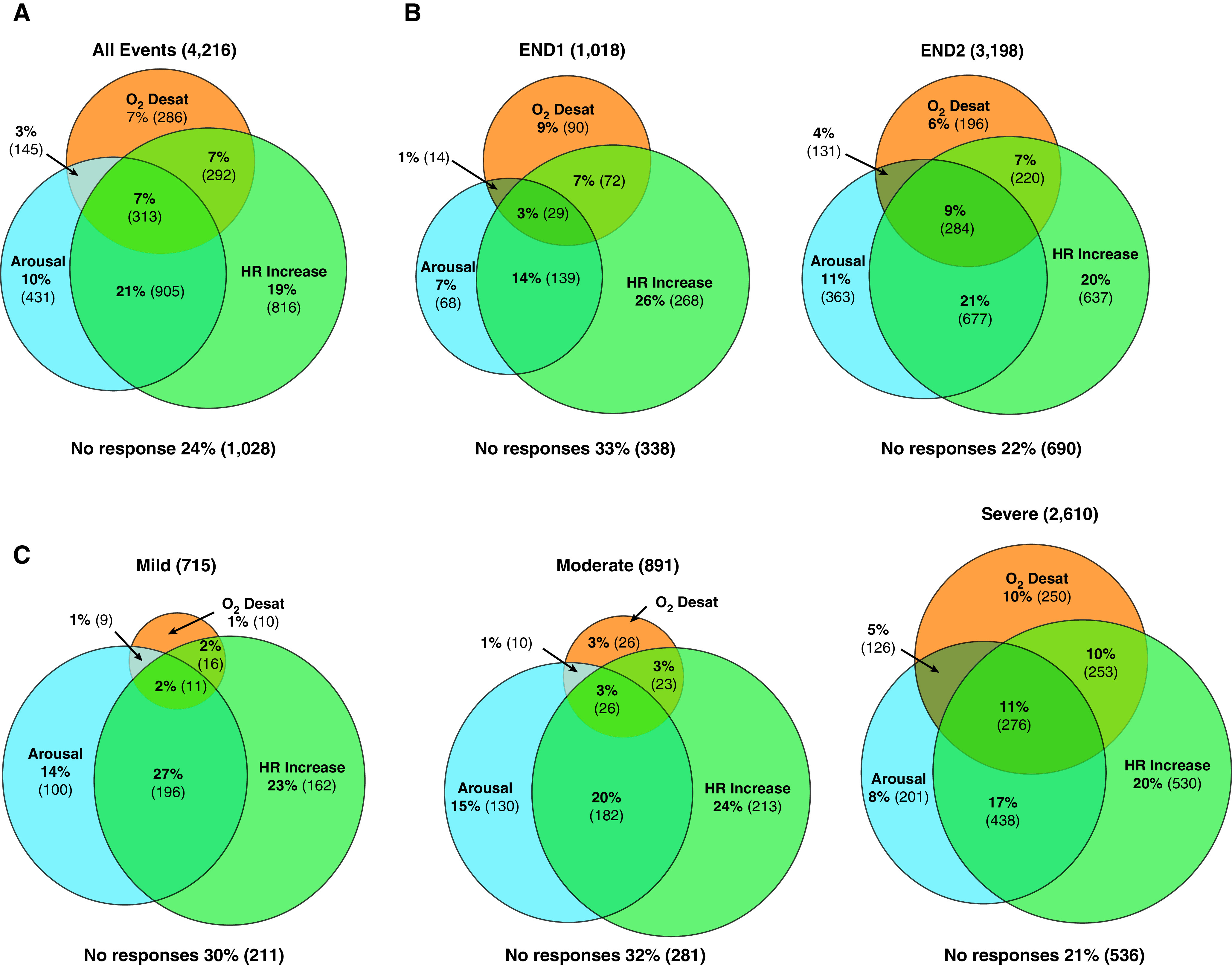
The frequency of possible combinations of desaturation, electroencephalography arousal, and increase in heart rate in (A) all events, (B) by END type, and (C) by degree of flow amplitude reduction. END1 = reappearance of one breath without clear flow limitation (typically sinusoidal) with an amplitude at least 200% of the amplitude of the two preceding breaths with inspiratory flow limitation; END2 = reappearance of at least two breaths without clear flow limitation (typically sinusoidal) of which at least one showed an amplitude at least 200% of the amplitude of the two preceding breaths with inspiratory flow limitation; Desat = desaturation; HR = heart rate.
Compared with IFL events that terminate with END1, there were more physiological responses associated with events that showed reappearance of two or more sinusoidal breaths at the termination of the IFL event (END2) (Figure 3B). END2 was associated with an increased likelihood of desaturation (odds ratio [OR], 1.57; 95% confidence interval [CI], 1.29–1.92), arousal (OR, 3.03; 95% CI, 2.54–3.61), and HR change (OR, 1.19; 95% CI, 1.01–1.40). However, the degree of flow reduction (mild, moderate, or severe) was not significantly associated with whether an EEG arousal or an increase in HR was present (Figure 3C). When compared with mild reduction (0–30%) in flow amplitude, a severe reduction (>50%) was more closely associated with desaturation (P < 0.01). When both the amplitude of flow reduction and the termination END1/END2 (i.e., Severe END2) were looked at, there was no combination that was consistently associated with any response (see Figure E1 in the online supplement). The frequency of the combinations of desaturation, EEG arousal, and increase in HR within each END type and the degree of flow amplitude reduction are shown by the rest of the Venn diagrams (Figures 4B and 4C).
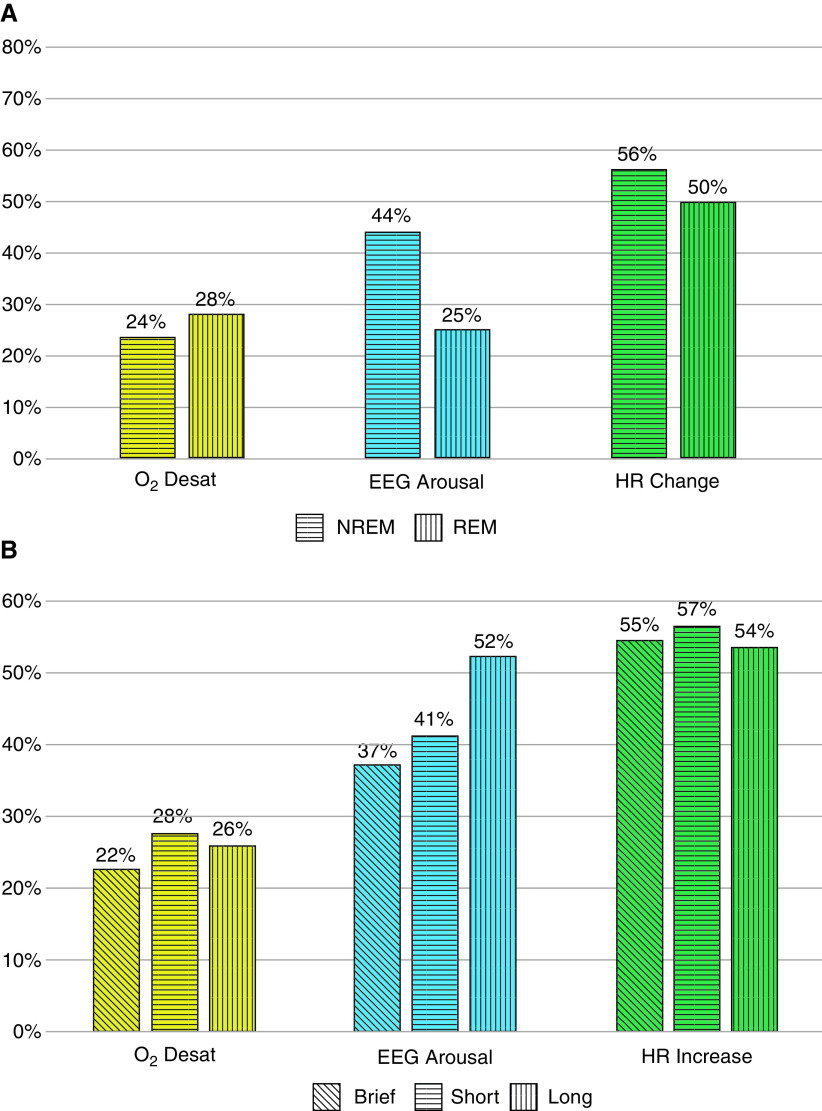
The percentages of severe END2 events associated with each consequence were evaluated separately by age, BMI, sex, REM/NREM sleep stages, sleepiness as measured by the ESS, and the duration of the IFL event. Age, BMI, sex, and sleepiness were not significantly associated with an increased frequency of either termination event or significantly associated with degree of flow amplitude reduction, and they were not significantly associated with a particular type of physiological response. REM sleep is associated with more END2 events (OR, 1.6; 95% CI, 1.3–2.0), more desaturations (OR, 2.0; 95% CI, 1.6–2.4), and fewer arousals (OR, 0.36; 95% CI, 0.3–0.44) (Figure 5A). Longer IFL events (>2 min) were significantly associated with more arousals than brief events (OR, 1.8; 95% CI, 1.5–2.3) (Figure 5B).
Figure 5.
Distribution of inspiratory flow limited events and responses by (A) sleep stage (rapid eye movement and non–rapid eye movement sleep) and (B) duration of inspiratory flow limited event (brief, 10–30 s; short, ⩾30–120 s; long, >120 s). Desat = desaturation; EEG = electroencephalography; HR = heart rate; NREM = non–rapid eye movement; REM = rapid eye movement.
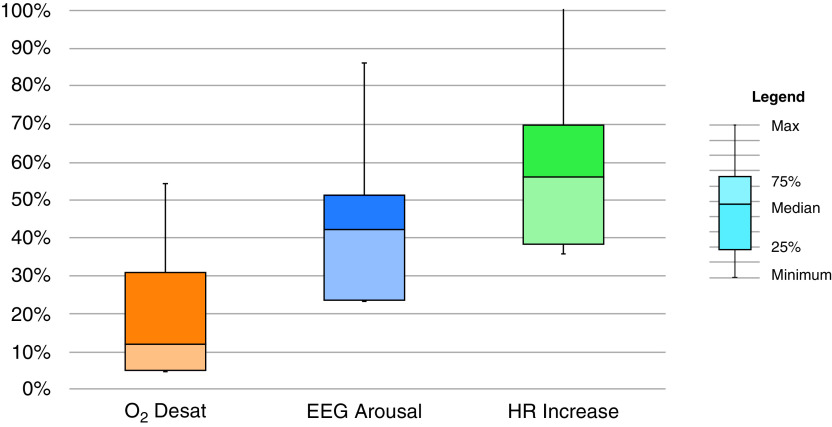
The median percentage of all IFL events in each individual subject associated with O2 desaturation, EEG arousal, and HR, respectively, are as follows: 12% ± 18% (range, 0–62%), 42% ± 19% (range 0–84%), and 57% ± 23% (range, 0–100%) (Figure 6). The median percentage of IFL events based on termination type and degree of flow amplitude reduction can be found in the online supplement (Figure E2).
Figure 6.
Median percentage of responses following all inspiratory flow limited events in individuals. The median percentage of all inspiratory flow limited events in each individual subject associated with O2 desaturation, electroencephalography arousal, and heart rate, respectively, are 12% ± 18% (range, 0–62%), 42% ± 19% (range, 0–84%), and 57% ± 23% (range, 0–100%). Desat = desaturation; EEG = electroencephalography; HR = heart rate; Max = maximum.
When a subject’s total number of END2 events is compared with their total number of hypopneas as defined by the AASM’s AHI3A, the Pearson correlation coefficient is 0.65 (P < 0.0001) (Figure E3).
Discussion
OSA is defined by a reduction in breathing that is self-limited because it produces physiological responses such as arousals, desaturation, and/or sympathetic activation. The exact nature of how to quantitate that disruption of breathing has evolved, with conflicting definitions. While our proposed definition may include events that are candidates to become hypopneas based on the AASM definition for hypopnea, our approach is novel in that it uses the consensus ATS algorithm for identifying IFL for each breath and adds a definition for the termination of IFL respiratory events.
Our interscorer percent agreement was high (>80%), comparable to several studies looking at the reliability of scoring other respiratory events during sleep (17, 18). This demonstrates the feasibility of manually scoring our novel IFL events based on our scoring rules.
Our study showed that IFL events that terminate with at least one of two normal sinusoidal breaths at least 200% of the baseline amplitude (END2) are associated with the most physiological responses, the most common of which is an increase in HR. This suggests to us the utility of this definition of an event to count. We also demonstrated that, whereas IFL events with a severe decrease in amplitude (>50%) are associated with more 3% O2 desaturations, there was no relationship between flow amplitude decrease and arousal/increase in HR. This suggests that the presence of IFL may capture more events with significant physiological responses than AASM-defined hypopneas, which require a >30% decrease in flow amplitude.
Although not all IFL events terminated in an EEG arousal according to AASM rules, this study confirms previous studies showing that IFL most commonly terminates with a form of arousal, either cortical or autonomic: 67% of all IFL events were associated with EEG arousal and/or an increase in HR. The lack of a strong association of event termination with 3-second EEG arousal may be secondary to our strict definition based on AASM criteria and does not limit the potential utility of our definition of an “event,” because our event scoring can now be studied for associations with other outcomes of OSA. Martin and colleagues demonstrated that nonvisible (autonomic) sleep fragmentation is associated with increased daytime sleepiness and impaired mood upon awakening (19). The formal AASM EEG Arousal definition has also been challenged by publications of Terzano and colleagues, who captured the clinical significance of cyclical alternating patterns of sleep (20).
Our data also show that immediate physiological responses to IFL events, including O2 desaturation, EEG arousal, and an increase in HR, can overlap but do not occur in a consistent hierarchical manner. This reaffirms results seen in our previous study that looked at respiratory events, such as apneas and hypopneas, overall (16). Although arousal from sleep has been shown to be associated with an autonomic response that includes an increase in blood pressure and HR, 10% of IFL events had only an arousal response alone without the HR response (21–23). Postevent tachycardia may be mediated by subcortical reflex responses, with only a portion contributed by EEG arousals (24). IFL events as defined by the present study were not consistently followed by a specific consequence. This implies that defining a significant IFL respiratory event by association to a single consequence, such as the AASM requirement of EEG arousal, will miss events that are associated with other meaningful responses (e.g., increase in HR and O2 desaturation). Therefore, it is unlikely that a hierarchical relationship can be calculated (i.e., no mathematical formula for “conversion” from one type of event consequence to another is likely to exist).
The different responses that result from IFL respiratory events may be further explained by variation in response among individuals (sex and age) or within individuals (sleep stage), or they may be dependent on IFL event duration. The present study addressed these possibilities by looking at subset analyses and found a lack of hierarchy of responses similar to the results of our pooled data and to our earlier study (13). Our study found that the association between IFL events and arousal (cortical or autonomic) was more common during NREM sleep compared with REM sleep. Also, we found that IFL events that resulted in a 3% O2 desaturation were more common in REM sleep, consistent with previous studies showing that obstructive apneas and hypopneas during REM sleep often led to greater hypoxemia (25). Some studies have shown that the duration of events was found to be a heritable phenotype, and patients with short-duration events were thought to have a lower arousal threshold (26–29). In contrast, our study found that IFL events of a longer duration (>2 min) were more likely to be associated with AASM-defined EEG arousal, whereas the occurrence of an increase in HR following an IFL termination was not predominant for any duration of IFL studied.
Our study found there to be variability among individual patients with respect to their physiological responses to IFL events. This may be due to the population of subjects that we studied (normal to mild OSA based on AHI4%), who may not behave like typical patients with severe OSA. The heterogeneity of these patients and their responses to IFL events may rely more on specific phenotypic complex qualities beyond the scope of our study.
Twenty-four percent of IFL events were not associated with any of the physiological responses we studied. While this can reflect the notion that there is a amount of IFL during sleep that is normal, we did not comprehensively study other acute responses (i.e., snoring, blood pressure increase) that may have occurred as a result of flow limitation. IFL may also play a role in stabilizing breathing while sleeping, but this may come at a physiologic cost. During sleep, the partial pressure of transcutaneous CO2 (tcco2) starts to increase until it reaches a plateau—a set point that provides target ventilation. Rimpilä and colleagues determined that an increase in flow-limited breaths associates with increased tcco2 rising to this set point. Persistent IFL can cause tcco2 to persist past this plateau and may result in adverse outcomes, including sympathetic overactivity and blood pressure variability (30). There is therefore a need to determine the threshold of IFL, as well as of IFL events, considered to be pathologic. A previous study looked at a population in Sao Paolo, Brazil, and determined that, epidemiologically, the 95th percentile value of IFL in apparently normal individuals was about 30% of breaths, suggesting that this percentage should be the upper limit of normal for subjects who do not have OSA (31). The approach in this last paper was, however, limited to the percentage of breaths with IFL rather than the number of “IFL events.”
Our study shows that IFL events defined by IFL with any reduction in flow and termination by at least two breaths without flow limitation and at least one breath with a 200% increase in amplitude resulted in the highest number of immediate physiological responses, i.e., EEG arousal, O2 desaturation, and HR increase. Currently, the sleep community assumes that these physiological responses define events that contribute to harder clinical outcomes, such as excessive daytime sleepiness, impaired neurocognitive function, and cardiovascular morbidity and mortality. While our study preliminarily looked at the relationship between ESS and our END2 events, we cannot conclude from our data that there is no relationship. Our sample size was too small for this purpose and was not powered to test long-term clinical outcomes. By using our method of identifying IFL events, further studies can be designed to robustly test the relationship of IFL events with long-term clinical outcomes, using the appropriate clinical metrics.
We also compared a subject’s total number of END2 events with their number of hypopneas as defined by AASM’s AHI3A in our 58 subjects. Although the results show a moderate correlation, they still deviate from the line of identity. This reinforces the potential utility of characterizing IFL events as distinct respiratory events, which can be examined more closely in a future study with a larger sample population.
The population most at risk for excluding flow limitation in AHI scoring are patients who have normal to mild OSA but still have persistent symptoms, particularly younger and pregnant patients. In a study looking at pediatric patients with sleep complaints and a pediatric AHI (AHI3A) of ⩽3, abnormal breathing patterns predominately consisting of flow limitation were seen with more frequency compared with an AASM-defined apnea and hypopnea and were associated with EEG disturbances (32). Pregnant patients with preeclampsia have been found to have a predominance of IFL with an improvement of blood pressure after CPAP use (33–35).
Our data support treating IFL in symptomatic patients even in the presence of a normalized AHI because of its affiliated physiological responses. Meurice and colleagues found that CPAP titration targeting the elimination of IFL as opposed to apneas/hypopneas/snoring led to an increase in sleep time and a more consistent improvement in maintenance of wakefulness testing (3). Despite our manual visualization of identifying IFL events using our formal set of rules, our interscorer agreement was high. Automation of this process of event detection during clinically polysomnography seems possible and will be key for larger-scale studies. It is already partially present in CPAP autotitrating algorithms programmed to treat IFL as obstructive events needing a rise in pressure (36, 37).
Although IFL—an imbalance between negative inspiratory and intraluminal pressure and activation of upper airway dilatory muscle—contributes to the development of sleep-disordered breathing events, it has been suggested that expiratory flow limitation could concomitantly occur with IFL and potentially contribute to the acute physiological responses seen after IFL events. However, isolated expiratory flow limitation during sleep is rare and was not the focus of our study (38).
Hosselet and colleagues incorporated flow limitation with apneas-hypopneas into an index (Respiratory Disturbance IndexTOTAL) that provided a better sensitivity and specificity for identifying subjects with excessive daytime sleepiness (39). In the present study, we did not adequately address the relationship among the IFL events that have specific responses to clinical outcomes, such as excessive daytime sleepiness and cardiovascular morbidity and mortality, because of the small sample size of patients. Our methodological approach for defining the events may contribute to future studies addressing these issues.
Polysomnography, the tool to diagnose OSA, allows us to perform more sophisticated recordings than ever and provides a plethora of data to the clinician, including measures of airflow, oxygen levels, and cardiac and skeletal muscle activity. Sleep-disordered breathing should not solely be measured by apneas and AASM hypopneas that end with either 3% desaturation or EEG arousal. There is an increasing emphasis on looking more closely at the broad range of the signals collected in the routine PSG to identify OSA subtypes in the hopes of defining personalized treatment of sleep-disordered breathing (40). Symptomatic patients who have normal to mild AHI may have predominant IFL during sleep.
This study demonstrates that IFL events are associated with physiological responses that plausibly could have clinical consequences. Further studies need to examine the contribution of IFL events with different responses to multiple possible clinical outcomes. We propose that providing a standardized definition of the “IFL event” (beyond identifying individual breaths with IFL) can lead to a standardized automation of scoring that includes IFL and will result in more vigorous studies looking at the significance of IFL as a respiratory event. The present study provides a framework for such a definition and will help in the execution of studies to define the effect of IFL on clinical outcomes as well as the incorporation of metrics of IFL into the diagnosis of sleep-disordered breathing.
Footnotes
Support by the Foundation for Research in Sleep Disorders and grants K24HL109156 and R01HL081310 from the National Heart, Lung, and Blood Institute of the National Institute of Health.
Author Contributions: J.T.G., A.P., I.A., and D.M.R. contributed to the conception and design of the work. J.T.G., B.C., H.J., A.P., I.A., and D.M.R. contributed to the acquisition, analysis, and interpretation of data. All authors revised the draft of the work critically. All authors approved the final version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Pevernagie DA, Gnidovec-Strazisar B, Grote L, Heinzer R, McNicholas WT, Penzel T, et al. On the rise and fall of the apnea-hypopnea index: a historical review and critical appraisal. J Sleep Res . 2020;29:e13066. doi: 10.1111/jsr.13066. [DOI] [PubMed] [Google Scholar]
- 2. Malhotra A, Ayappa I, Ayas N, Collop N, Kirsch D, McArdle N, et al. Metrics of sleep apnea severity: beyond the AHI. Sleep (Basel) . 2021;44:zsab030. doi: 10.1093/sleep/zsab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meurice JC, Paquereau J, Denjean A, Patte F, Series F. Influence of correction of flow limitation on continuous positive airway pressure efficiency in sleep apnoea/hypopnoea syndrome. Eur Respir J . 1998;11:1121–1127. doi: 10.1183/09031936.98.11051121. [DOI] [PubMed] [Google Scholar]
- 4. Condos R, Norman RG, Krishnasamy I, Peduzzi N, Goldring RM, Rapoport DM. Flow limitation as a noninvasive assessment of residual upper-airway resistance during continuous positive airway pressure therapy of obstructive sleep apnea. Am J Respir Crit Care Med . 1994;150:475–480. doi: 10.1164/ajrccm.150.2.8049832. [DOI] [PubMed] [Google Scholar]
- 5. Hosselet JJ, Norman RG, Ayappa I, Rapoport DM. Detection of flow limitation with a nasal cannula/pressure transducer system. Am J Respir Crit Care Med . 1998;157:1461–1467. doi: 10.1164/ajrccm.157.5.9708008. [DOI] [PubMed] [Google Scholar]
- 6. Ayappa I, Norman RG, Krieger AC, Rosen A, O’Malley RL, Rapoport DM. Non-invasive detection of respiratory effort-related arousals (REras) by a nasal cannula/pressure transducer system. Sleep . 2000;23:763–771. doi: 10.1093/sleep/23.6.763. [DOI] [PubMed] [Google Scholar]
- 7. Pamidi S, Redline S, Rapoport D, Ayappa I, Palombini L, Farre R, et al. American Thoracic Society Ad Hoc Committee on Inspiratory Flow Limitation An Official American Thoracic Society Workshop Report: noninvasive identification of inspiratory flow limitation in sleep studies. Ann Am Thorac Soc . 2017;14:1076–1085. doi: 10.1513/AnnalsATS.201704-318WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness: the upper airway resistance syndrome. Chest . 1993;104:781–787. doi: 10.1378/chest.104.3.781. [DOI] [PubMed] [Google Scholar]
- 9. Guilleminault C, Poyares D, Rosa Ad, Kirisoglu C, Almeida T, Lopes MC. Chronic fatigue, unrefreshing sleep and nocturnal polysomnography. Sleep Med . 2006;7:513–520. doi: 10.1016/j.sleep.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 10. Pelin Z, Karadeniz D, Oztürk L, Gözükirmizi E, Kaynak H. The role of mean inspiratory effort on daytime sleepiness. Eur Respir J . 2003;21:688–694. doi: 10.1183/09031936.03.00298903. [DOI] [PubMed] [Google Scholar]
- 11. Pépin JL, Guillot M, Tamisier R, Lévy P. The upper airway resistance syndrome. Respiration . 2012;83:559–566. doi: 10.1159/000335839. [DOI] [PubMed] [Google Scholar]
- 12. Pamidi S, Pinto LM, Marc I, Benedetti A, Schwartzman K, Kimoff RJ. Maternal sleep-disordered breathing and adverse pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol . 2014;210:52.e51–52.e14. doi: 10.1016/j.ajog.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 13. McGinley B, Halbower A, Schwartz AR, Smith PL, Patil SP, Schneider H. Effect of a high-flow open nasal cannula system on obstructive sleep apnea in children. Pediatrics . 2009;124:179–188. doi: 10.1542/peds.2008-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Young LR, Taxin ZH, Norman RG, Walsleben JA, Rapoport DM, Ayappa I. Response to CPAP withdrawal in patients with mild versus severe obstructive sleep apnea/hypopnea syndrome. Sleep (Basel) . 2013;36:405–412. doi: 10.5665/sleep.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parekh A, Mullins AE, Kam K, Varga AW, Rapoport DM, Ayappa I. Slow-wave activity surrounding stage N2 K-complexes and daytime function measured by psychomotor vigilance test in obstructive sleep apnea. Sleep (Basel) . 2019;42:zsy256. doi: 10.1093/sleep/zsy256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ayappa I, Rapaport BS, Norman RG, Rapoport DM. Immediate consequences of respiratory events in sleep disordered breathing. Sleep Med . 2005;6:123–130. doi: 10.1016/j.sleep.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 17. Whitney CW, Gottlieb DJ, Redline S, Norman RG, Dodge RR, Shahar E, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep . 1998;21:749–757. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 18. Rosenberg RS, Van Hout S. The American Academy of Sleep Medicine Inter-scorer Reliability program: respiratory events. J Clin Sleep Med . 2014;10:447–454. doi: 10.5664/jcsm.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin SE, Wraith PK, Deary IJ, Douglas NJ. The effect of nonvisible sleep fragmentation on daytime function. Am J Respir Crit Care Med . 1997;155:1596–1601. doi: 10.1164/ajrccm.155.5.9154863. [DOI] [PubMed] [Google Scholar]
- 20. Terzano MG, Parrino L, Smerieri A, Chervin R, Chokroverty S, Guilleminault C, et al. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med . 2002;3:187–199. doi: 10.1016/s1389-9457(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 21. Horner RL, Brooks D, Kozar LF, Tse S, Phillipson EA. Immediate effects of arousal from sleep on cardiac autonomic outflow in the absence of breathing in dogs. J Appl Physiol (1985) . 1995;79:151–162. doi: 10.1152/jappl.1995.79.1.151. [DOI] [PubMed] [Google Scholar]
- 22. Sforza E, Jouny C, Ibanez V. Cardiac activation during arousal in humans: further evidence for hierarchy in the arousal response. Clin Neurophysiol . 2000;111:1611–1619. doi: 10.1016/s1388-2457(00)00363-1. [DOI] [PubMed] [Google Scholar]
- 23. Azarbarzin A, Ostrowski M, Hanly P, Younes M. Relationship between arousal intensity and heart rate response to arousal. Sleep (Basel) . 2014;37:645–653. doi: 10.5665/sleep.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Azarbarzin A, Ostrowski M, Moussavi Z, Hanly P, Younes M. Contribution of arousal from sleep to postevent tachycardia in patients with obstructive sleep apnea. Sleep (Basel) . 2013;36:881–889. doi: 10.5665/sleep.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Findley LJ, Wilhoit SC, Suratt PM. Apnea duration and hypoxemia during REM sleep in patients with obstructive sleep apnea. Chest . 1985;87:432–436. doi: 10.1378/chest.87.4.432. [DOI] [PubMed] [Google Scholar]
- 26. Liang J, Cade BE, Wang H, Chen H, Gleason KJ, Larkin EK, et al. Comparison of heritability estimation and linkage analysis for multiple traits using principal component analyses. Genet Epidemiol . 2016;40:222–232. doi: 10.1002/gepi.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sforza E, Boudewijns A, Schnedecker B, Zamagni M, Krieger J. Role of chemosensitivity in intrathoracic pressure changes during obstructive sleep apnea. Am J Respir Crit Care Med . 1996;154:1741–1747. doi: 10.1164/ajrccm.154.6.8970364. [DOI] [PubMed] [Google Scholar]
- 28. Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep . 1997;20:654–675. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- 29. Sands SA, Terrill PI, Edwards BA, Taranto Montemurro L, Azarbarzin A, Marques M, et al. Quantifying the arousal threshold using polysomnography in obstructive sleep apnea. Sleep (Basel) . 2018;41:zsx183. doi: 10.1093/sleep/zsx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rimpilä V, Saaresranta T, Huhtala H, Virkki A, Salminen AV, Polo O. Transcutaneous CO(2) plateau as set-point for respiratory drive during upper airway flow-limitation. Respir Physiol Neurobiol . 2014;191:44–51. doi: 10.1016/j.resp.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 31. Palombini LO, Tufik S, Rapoport DM, Ayappa IA, Guilleminault C, de Godoy LB, et al. Inspiratory flow limitation in a normal population of adults in São Paulo, Brazil. Sleep (Basel) . 2013;36:1663–1668. doi: 10.5665/sleep.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guilleminault C, Huang YS, Chin WC, Okorie C. The nocturnal-polysomnogram and “non-hypoxic sleep-disordered-breathing” in children. Sleep Med . 2019;60:31–44. doi: 10.1016/j.sleep.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 33. Edwards N, Blyton DM, Kirjavainen T, Kesby GJ, Sullivan CE. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med . 2000;162:252–257. doi: 10.1164/ajrccm.162.1.9905006. [DOI] [PubMed] [Google Scholar]
- 34. Connolly G, Razak AR, Hayanga A, Russell A, McKenna P, McNicholas WT. Inspiratory flow limitation during sleep in pre-eclampsia: comparison with normal pregnant and nonpregnant women. Eur Respir J . 2001;18:672–676. doi: 10.1183/09031936.01.00053501. [DOI] [PubMed] [Google Scholar]
- 35. Poyares D, Guilleminault C, Hachul H, Fujita L, Takaoka S, Tufik S, et al. Pre-eclampsia and nasal CPAP: part 2. Hypertension during pregnancy, chronic snoring, and early nasal CPAP intervention. Sleep Med . 2007;9:15–21. doi: 10.1016/j.sleep.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 36. Berry RB, Kushida CA, Kryger MH, Soto-Calderon H, Staley B, Kuna ST. Respiratory event detection by a positive airway pressure device. Sleep (Basel) . 2012;35:361–367. doi: 10.5665/sleep.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li QY, Berry RB, Goetting MG, Staley B, Soto-Calderon H, Tsai SC, et al. Detection of upper airway status and respiratory events by a current generation positive airway pressure device. Sleep (Basel) . 2015;38:597–605. doi: 10.5665/sleep.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stănescu D, Kostianev S, Sanna A, Liistro G, Veriter C. Expiratory flow limitation during sleep in heavy snorers and obstructive sleep apnoea patients. Eur Respir J . 1996;9:2116–2121. doi: 10.1183/09031936.96.09102116. [DOI] [PubMed] [Google Scholar]
- 39. Hosselet J, Ayappa I, Norman RG, Krieger AC, Rapoport DM. Classification of sleep-disordered breathing. Am J Respir Crit Care Med . 2001;163:398–405. doi: 10.1164/ajrccm.163.2.9808132. [DOI] [PubMed] [Google Scholar]
- 40. Mazzotti DR, Lim DC, Sutherland K, Bittencourt L, Mindel JW, Magalang U, et al. Opportunities for utilizing polysomnography signals to characterize obstructive sleep apnea subtypes and severity. Physiol Meas . 2018;39:09TR01. doi: 10.1088/1361-6579/aad5fe. [DOI] [PMC free article] [PubMed] [Google Scholar]