Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic is responsible for an important global death toll from which sub-Saharan Africa (SSA) seems mostly protected. The reasons explaining this situation are still poorly understood.
Methods
We analyzed the correlation between reported COVID-19 data between February 14, 2020 and May 18, 2021, and demographic, socioeconomic, climatic, diagnostic data, and comorbidities in 47 SSA countries. Different databases including the WHO data center, Our World in Data, and the World Bank were used.
Findings
As of May 17, 2021, SSA reported 2% of COVID-19 cases and 2.9% of deaths, with the southern region being the most affected with 56.4% of cases and 75.0% of deaths. COVID-19 mortality was positively correlated with medical variables (national obesity rate, diabetes prevalence, cancer incidence, and cardiovascular disease mortality rate), socioeconomic characteristics (international tourism, per capita health expenditure, human development index, HDI, and years of schooling), and health system variables (nurse density, number of COVID-19 tests per capita), but negatively correlated with the population under 15 years of age and the malaria index.
Interpretation
Our study suggests that higher economic status fits with high COVID-19 mortality in SSA. In this regard, it represents primarily a disease of modern and wealthy societies, and can therefore be considered as an exception among infectious diseases that historically affected more severely underserved populations living in low- and middle-income countries. However, it should be made clear that observed correlations do not imply inevitably causation and that additional studies are necessary to confirm our observations.
Keywords: COVID-19, Sub-Saharan Africa, Countries, Risk factors, Mortality
Abbreviations: SSA, Sub-Saharan Africa
Highlights
-
•
Obesity, diabetes , and socio-economic indicators of affluence and better development correlate significantly with an increased COVID-19 mortality in May 2021.
-
•
The youth of the sub-Saharan population and diseases affecting primarily children, such as malaria, correlate significantly with lower COVID-19 mortality in May 2021.
-
•
In Sub-Saharan Africa, COVID-19 is an infectious disease with a unique pattern as it affects primarily the richest nations.
COVID-19; Sub-Saharan Africa; Countries; Risk factors; Mortality.
1. Introduction
Severe Acute Respiratory Syndrome CoronaVirus-2 (SARS-CoV-2) was detected for the first time in Wuhan, in late 2019. This virus is the cause of the coronavirus disease 2019 (COVID-19) pandemic that had a dramatic impact around the world [1]. As of August 15, 2022, SARS-CoV-2 has infected over 0.591 billion or 7.6% out of 7.7 billion humans [2]. More importantly, it has caused the death of 6.4 million people (1% mortality rate) [2].
A surprising feature of the COVID-19 pandemic, to date, is the mildness of its reported impact in sub-Saharan African (SSA) countries compared to other regions of the world despite its spread to almost all African countries in less than three months in 2020 [3].
As of August 15, 2022, the number of reported cases and deaths in sub-Saharan Africa is much lower than in other geographic regions [2]. All 47 SSA countries considered in our study combined reported 770 cases and 15 deaths per 100,000 people [2]. The same is true for the timeframe considered in our analyses. Indeed, by mid-May 2021, all 47 countries in SSA reported 303 cases and 7.6 deaths per 100,000 people [2]. These rates are much lower than those observed over the same period in the rest of the world. The most commonly cited potential explanations to these differences include warmer climate [4], younger population [5], poorly identified genetic factors [6], lower rates of co-morbidities on the African continent, the presence of SARS-CoV-2-like animal coronaviruses in the local fauna, induced immunity from Bacillus Calmette-Guérin (BCG) vaccination, and many others [6]. With the ongoing emergence of SARS-CoV-2 variants, it remains uncertain whether SSA, excluding South Africa, will remain mildly affected. Indeed, the largest part of SSA appears to be escaping the COVID-19 despite health systems considered as fragile [6,7].
To shed light on this conundrum, we decided to explore available data. Originally struck by the above-mentioned specificity of the pandemic in the countries of SSA when compared to other regions of the world, we formulated the hypothesis that such a massive phenomenon on the scale of a continent could be linked to demographic, epidemiological, climatic, or even economic characteristics representing some of the hallmarks of the continent. In addition, we reasoned that the quantitative estimates of these features would, for most of them, not be hidden or “cryptic” but rather relatively easy to obtain from different databases and will provide us with reasonably plausible explanations concerning the huge spread of COVID-19 mortality observed between sub-Saharan African nations (on May 17, 2021, mortality in South Africa was 275-fold higher than in Eritrea). Finally, we hypothesized that both the large number and the relative diversity of SSA countries would help us through the inherent modulation of these characters between countries as well as through statistical depth (47 countries) and would generate significant correlations between mortality from COVID-19 and some of the variables examined. In this paper, we examined the heterogeneous spread and severity of COVID-19 in sub-Saharan Africa, described the epidemiology of COVID-19 in SSA and in each region of SSA as of May 18, 2021 using data retrieved from the Johns Hopkins Coronavirus Resource Center platform (https://coronavirus.jhu.edu/map.html). We then performed a statistical analysis of the relationship between putative indicators of the spread and mortality from COVID-19 and publicly available socioeconomic, demographic, epidemiologic, and climatic aspects.
2. Methodologies
2.1. Study design and data sources
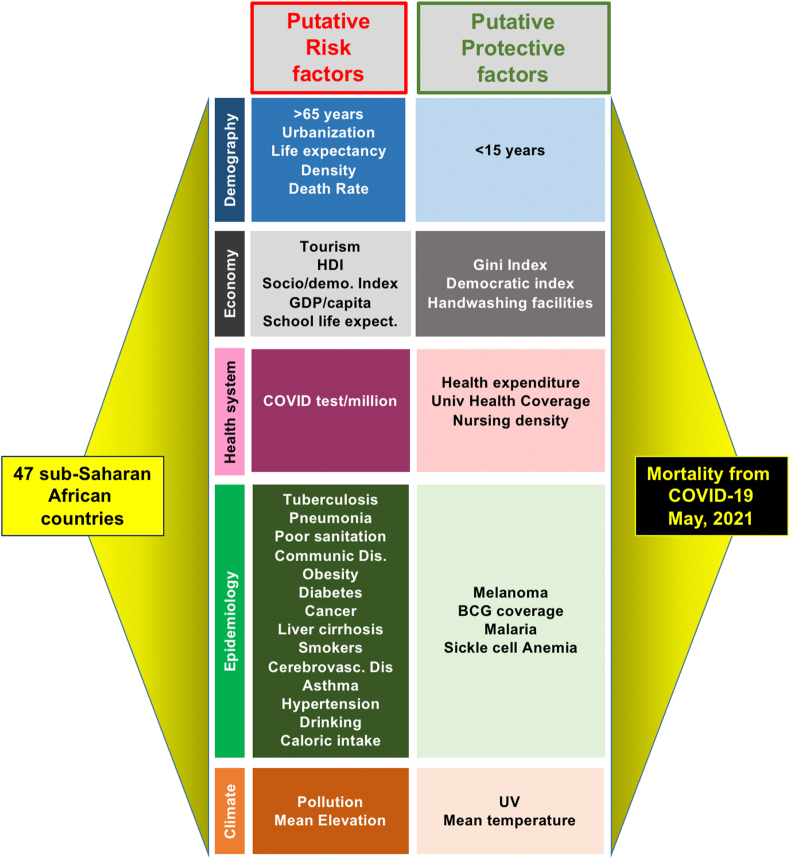
This is a cross-sectional analysis of aggregate epidemiological data (confirmed COVID-19 cases, deaths, and tests performed) collected between February 14, 2020, and May 17, 2021, on the Johns Hopkins Coronavirus Resource Center platform (https://coronavirus.jhu.edu/map.html) for 47 SSA countries (Burundi and Tanzania were excluded due to lack of available data, see Supplemental Table S1). Overall, to conduct this research we tried to conform with the PICO (Population, Intervention or Exposure, Comparison, Outcome) framework elements applicable to etiological studies [8]. The Population studied was that of the 47 countries located in SSA. The Exposures were the 47 different putative variables correlated with COVID-19 mortality (see Figure 1). Comparisons were made between the different SSA countries. The Outcome was the mortality rate in each country.8Molecular and antigenic test data were verified by direct reports from member states to CDC Africa. For global epidemiological data comparisons, official WHO daily situation reports and the WHO COVID-19 dashboard were used as references. Demographic, epidemiological, socioeconomic, health systems, and climate data were provided by the International Agency for Research on Cancer (https://www.iarc.who.int/), Our World in Data (https://ourworldindata.org/), the World Bank (https://databank.worldbank.org/databases), WHO (https://apps.who.int/nha/database/Home/Index/fr), and the Climate Data Store (https://fr.climate-data.org/), respectively.
Figure 1.
Heuristic approach employed to assess the importance of a collection of relevant variables on mortality from COVID-19 in 47 SSA countries.
2.2. Statistical analysis
Descriptive analyses were conducted to establish cumulative cases, cumulative incidence (cases per 100,000 population), case-fatality ratio (CFR; number of reported deaths per number of reported cases × 100), test ratio (number of tests per 1 million individuals), and tests per case. Population estimates for 2019 are from the United Nations Population Fund (www.unfpa.org/data/world-population-dashboard). We calculated Pearson's and Spearman's coefficients and p-values between the COVID-19 mortality rate (as of May 18, 2021) and variables selected for their apparent relevance to the disease. Our analysis included a total of 47 variables: 7 demographic, 4 health systems, 10 socioeconomic, 3 climatic, and 23 epidemiological (Table 1). We focused primarily in this work on the data provided by the Spearman's r correlation coefficient, because it measures nonparametric statistical dependence between two variables. All correlation coefficients and p-values were calculated using Prism 9.3.0 release [9].
Table 1.
The different parameters analyzed with their Pearson and Spearman coefficients and corresponding P values.
| Variable | Category | p-value | Pearson r | p-value | Spearman r |
|---|---|---|---|---|---|
| Exposure to solar ultraviolet (UV) radiation, Data by country, 2004 | Climate | 0.00036 | −0.49367 | 0.05815 | −0.27540 |
| Mean Temperature in °C | Climate | 0.01099 | −0.36392 | 0.12703 | −0.22333 |
| Ambient and household air pollution death rate per 100,000, 2016 | Climate | 0.01570 | −0.34338 | 0.00131 | −0.44604 |
| Mean elevation by countries (m) | Climate | 0.86369 | 0.02544 | 0.47125 | −0.10649 |
| Pop. >65 (%), 2019 | Demography | 0.02801 | 0.31399 | 0.00171 | 0.43644 |
| Pop. <15 (%), 2019 | Demography | 0.00013 | −0.51846 | 0.00000 | −0.60379 |
| Urbanization (%), 2018 | Demography | 0.04648 | 0.28583 | 0.00352 | 0.40903 |
| Life expectancy (years) 2019 | Demography | 0.08383 | 0.24949 | 0.01956 | 0.33255 |
| Population density (hab/km2), 2021 | Demography | 0.68107 | −0.0602 | 0.57854 | −0.08132 |
| Crude death rate per 1,000 people, 2019 | Demography | 0.87977 | −0.02242 | 0.13472 | −0.21903 |
| Health expenditure/capita, 2019 | Health system | 0.00000 | 0.60496 | 0.00000 | 0.63247 |
| Universal Health Service Coverage index, WHO 2019 | Health system | 0.00000 | 0.62891 | 0.00014 | 0.51715 |
| Nursing and Midwifery personnel density per 1,000 people, 2019 | Health system | 0.00000 | 0.67443 | 0.00047 | 0.48505 |
| COVID-19 tests/inhabitant, May 2021 | Health system | 0.00002 | 0.57086 | 0.00000 | 0.67252 |
| Incidence of tuberculosis per 100,000 per year, 2020 | Medical | 0.07258 | 0.25877 | 0.42435 | 0.11674 |
| Population attributable fraction Melanoma UV, 2004 | Medical | 0.03012 | 0.35218 | 0.80674 | 0.04103 |
| Deaths from pneumonia per1000 people, 2017 | Medical | 0.08311 | −0.25006 | 0.00567 | −0.38944 |
| BCG coverage (%), 2019 | Medical | 0.11365 | 0.22889 | 0.01881 | 0.33451 |
| Deaths due to tuberculosis among HIV-negative patients per 100,000 people, WHO, 2020 | Medical | 0.76651 | 0.04352 | 0.54492 | 0.08860 |
| Disability-adjusted life years (DALYs) lost to communicable diseases per 100,000 people, 2017 | Medical | 0.06822 | −0.26268 | 0.00073 | −0.46632 |
| Deaths from pneumonia, 2017 | Medical | 0.39612 | −0.12395 | 0.00014 | −0.51632 |
| Mortality rate from unsafe water/sanitation and lack of hygiene (WASH), 2016 | Medical | 0.00149 | −0.44152 | 0.00000 | −0.61091 |
| Share of Disease burden from Communicable diseases, 2017 | Medical | 0.00808 | −0.37416 | 0.00000 | −0.62367 |
| Malaria estimates, cases per million, 2017 | Medical | 0.12747 | −0.22551 | 0.00000 | −0.68638 |
| Obesity =(%), 2016 | Medical | 0.0000 | 0.82431 | 0.00000 | 0.63394 |
| Raised blood Glucose>7 mmol/l, 2014 | Medical | 0.00118 | 0.45408 | 0.00000 | 0.61562 |
| Deaths from Cardiovascular diseases - (%), 2017 | Medical | 0.03132 | 0.30799 | 0.00001 | 0.57826 |
| Cancer incidence, crude rate, 2020 | Medical | 0.00020 | 0.51169 | 0.00004 | 0.55398 |
| Cancer incidence, age-standardized rate, 2020 | Medical | 0.00063 | 0.47534 | 0.12574 | 0.22407 |
| Liver cirrhosis death rates (>15) per 100,000 females, 2016 | Medical | 0.00490 | −0.39555 | 0.00417 | −0.40213 |
| Newborns with Sickle Cell Anemia/million people, 2010 | Medical | 0.00631 | −0.39279 | 0.00329 | −0.41991 |
| Liver cirrhosis death rates (>15) per 100,000 males, 2016 | Medical | 0.04801 | −0.28394 | 0.06572 | −0.26502 |
| Estimated prevalence of daily smokers, 2012 | Medical | 0.10040 | 0.23997 | 0.05939 | 0.27409 |
| Deaths from Cerebrovascular diseases, Age-standardized (rate per 100,000), 2017 | Medical | 0.10789 | −0.23251 | 0.17752 | −0.19581 |
| Prevalence of Asthma - Age-standardized (%), 2017 | Medical | 0.11127 | −0.23037 | 0.56331 | −0.08459 |
| Raised blood pressure>140-90, 2015 | Medical | 0.38030 | 0.12951 | 0.61601 | 0.07424 |
| Daily caloric supply, Kcal (FAO,2017 ) | Medical | 0.82643 | −0.03579 | 0.66614 | −0.07036 |
| Heavy episodic drinking, age-standardized (>15, 2016) | Medical | 0.84424 | 0.02911 | 0.81436 | −0.03479 |
| Newborns with Sickle Cell Anemia, 2010 | Medical | 0.26582 | −0.16564 | 0.00001 | −0.58322 |
| Ratio million tourist/million inhabitants, 2017 | Socio-economic | 0.00493 | 0.41192 | 0.00000 | 0.64701 |
| Human Development Index (HDI), 2020 | Socio-economic | 0.00006 | 0.54551 | 0.00000 | 0.61784 |
| Socio-demographic Index (SDI), The Lancet, 2019 | Socio-economic | 0.00020 | 0.50590 | 0.00000 | 0.59992 |
| GDP per capita in dollars, 2019 | Socio-economic | 0.00150 | 0.44126 | 0.00001 | 0.57408 |
| School life expectancy (years) 2017 | Socio-economic | 0.00008 | 0.53724 | 0.00019 | 0.51255 |
| International tourism, number of arrivals, in million, 2017 | Socio-economic | 0.00000 | 0.68700 | 0.38067 | 0.13386 |
| Index of Gini, 2019 | Socio-economic | 0.00117 | 0.45906 | 0.02931 | 0.31812 |
| Democratic Index (The Economist), 2020 | Socio-economic | 0.00949 | 0.38259 | 0.12631 | 0.23130 |
| Basic handwashing facilities including soap and water (% of population), 2017 | Socio-economic | 0.08395 | 0.27666 | 0.16264 | 0.22505 |
Principal component analysis was performed on R (4.1.1) and RStudio (1.4.1717), using the FactoMineR (2.4) and factoextra (1.0.7) packages. Variables used are listed in Supplemental Table 2. SSA region and COVID-19 mortality rate on May 17, 2021 were used as supplementary variables. All 47 SSA countries except Nigeria and Democratic Republic of the Congo were included, because the high levels of malaria, pneumonia, and sickle cell anemia in both countries made the PCA figure difficult to read. Missing values were imputated with a PCA model at two dimensions, using the missMDA package (1.18). Only countries with cos2 > 0.1 were displayed. African maps were realized on R (version 4.1.1) and RStudio (1.4.1717), using the ggplot2 (3.3.5), ggmap (3.0.0), maps (3.4.0), and dplyr (1.0.7) packages. Partial Least Square analysis was performed on R (4.1.1) and RStudio (1.4.1717), using the ropls (1.26.4) package. Variables used are listed in Supplemental Table 3. Missing values were imputed with a PCA model at two dimensions, using the missMDA package (1.18). Hierarchical clustering was performed using ClustVis (https://biit.cs.ut.ee/clustvis/), a web-based tool for visualizing clustering of multivariate data. Data were clustered according to average Euclidian distances for rows (countries) and average correlation for columns (analyzed parameters).
3. Results
-
•
EPIDEMIOLOGICAL SITUATION
As of May 18, 2021, 3,283,371 cases and 84,289 deaths related to COVID-19 have been reported from the 47 SSA countries, representing 2% of the 166,352,007 cases and 2.9% of the 2,908,836 deaths reported worldwide [3].
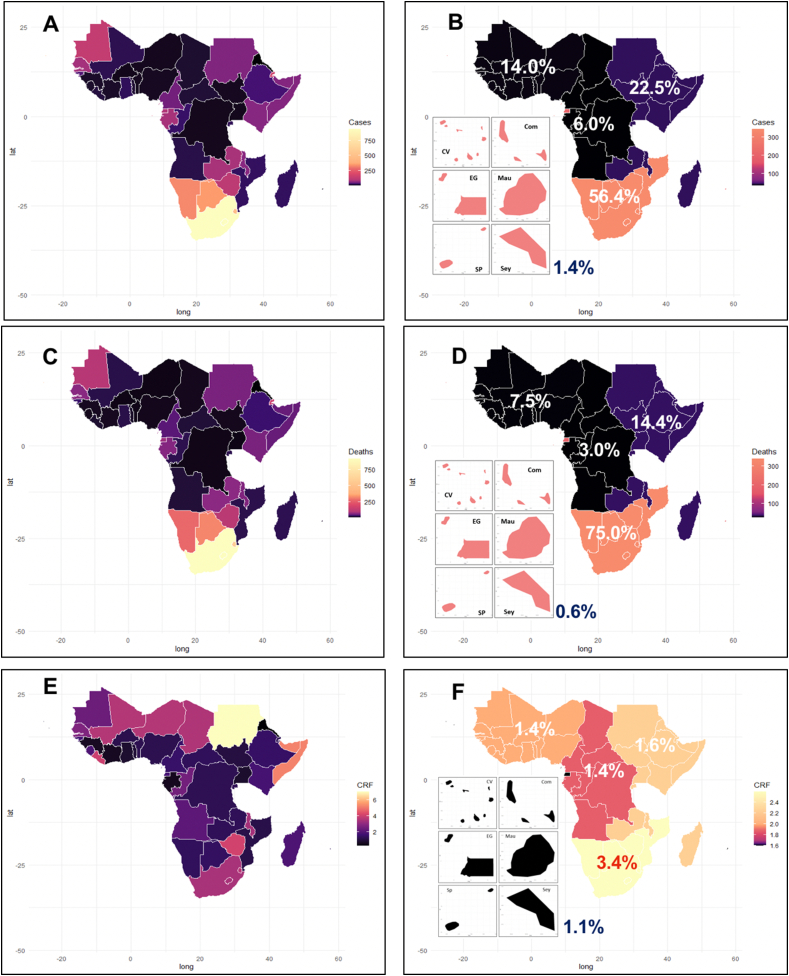
Three quarters (n = 35) of these 47 countries detected their first cases very early, between February 14 and March 21, 2020. Thereafter, the intensity of the epidemic varied greatly across SSA (Figure 2A, B, C & D): 56.4% of cases and 75% of deaths occurred in the Southern region, while the corresponding figures ranged from 1.4% to 22.6% and from 0.6% to 14.4% in other SSA regions (regions are defined in Supplemental Table 1, Figure 2 B & D). Eight countries accounted for 77.3% (2,538,851) of reported COVID-19 cases in SSA: South Africa reported 49% of the total, while the remaining seven countries (Ethiopia, Kenya, Nigeria, Ghana, Zambia, Cameroon, and Mozambique) reported between 2.0 and 7.0% of cases. The remaining 39 countries reported between 1,288 and 52,162 cases. Concerning fatalities, 75.6% stemmed from five countries (Ethiopia, Kenya, Sudan, Nigeria, and South Africa), with 62% from South Africa. Thus, while South Africa suffered disproportionately from the early waves of the pandemic, Nigeria was largely spared with a population 3.8 times larger but 32 times fewer COVID-19-related deaths, meaning that mortality in South Africa was, as of May 2021, 125 times higher than in Nigeria.
Figure 2.
Epidemiological situation of COVID-19 in SSA in May 2021. A: cumulative number of cases per country; B: cumulative number (%) of cases per region; C: cumulative number of deaths per country; D: cumulative number (%) of deaths per region; E: COVID-19 case fatality rate per country; F: case fatality rate (%) per region. Panels of six maps in B, D, and F correspond to insular countries around the continent.
Altogether SSA countries maintained a mean case fatality rate (CFR) of 2.5% since August 2020 [10]. A subset of 13 countries (Sudan, Somalia, Zimbabwe, Liberia, Comoros, Eswatini, Chad, South Africa, Malawi, Gambia, and Senegal) reported CFR between 2.7% and 6.7% i.e. above the mean value. The Southern region reported the highest regional CFR (3.4%) of SSA, making other regions looking protected in comparison (Figure 2 E & F). Likewise, in smaller insular nations (Seychelles, Comoros, Mauritius, Sao Tome, Cape Verde; Madagascar was not included), death rates were comparatively higher than on the continent taken as a whole (Figure 2F).
Mortality by COVID-19 is particularly clear at the sub-continental level. As of May 17th, 2021, among the ten most afflicted nations in SSA, six countries are in Southern Africa (Namibia, South Africa, Botswana, Eswatini, Zimbabwe, and Lesotho) and three are insular (Seychelles, Cape Verde, and Sao Tome and Principe). Four of these countries (Namibia, South Africa, Seychelles, and Botswana) displayed more than 1,000 deaths per million (1,007 to 1,521), comparable to rates prevailing in Europe or America, but considerably higher than those observed in Asia.
-
●
HEALTH SYSTEM INDICATORS
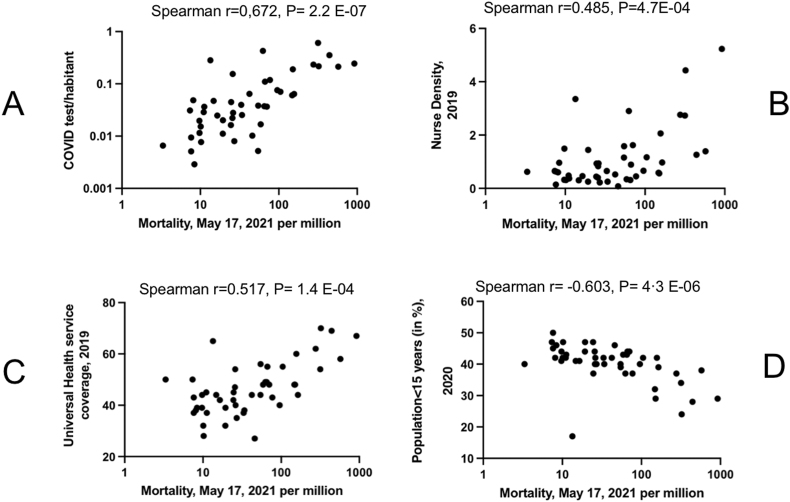
We next investigated the factors explaining such a wide disparity in SSA. We first explored the impact of national diagnostic capacities known to increase the knowledge about the virus circulation and its impact on the populations [11–13]. We could observe a positive correlation (Spearman r = 0.672, P = 2.2 E−07) between the number of tests per inhabitant and COVID-19 national mortalities in SSA (Figure 3A). Although testing rate may be low in some nations due to a low circulation of the virus, our observation implies that COVID-19 fatalities might have been underestimated in some countries due to the lack of diagnostic capacities or political will. As an illustration, Botswana, the best tester in SSA (0.67 test/inhabitant) ranks 4th for mortality while the Democratic Republic of Congo, the worst tester, ranks 44 (out of 49) on May 17th, 2021.
Figure 3.
Dot plots corresponding to the correlation between COVID-19 mortality and health system or demographic indicators. A- Correlation of COVID-19 mortality rate with the number of PCR tests performed per inhabitant in each SSA country. B-Correlation of COVID-19 mortality rate in SSA countries with nurse density. C- Correlation of COVID-19 mortality rate in SSA with the universal health service coverage. D-Correlation of COVID-19 mortality rate in SSA with the percentage of the population in SSA under 15 years old.
The rapid growth of COVID-19 cases has challenged the national capacity of health systems worldwide [12,14]. To estimate the extent of the phenomenon in SSA, we tested the correlation between a few important health system indicators and COVID-19 mortality (Figure 3B and C). Two parameters were strongly correlated with COVID-19 mortality: nurse density (Spearman r = 0.485, P = 0.0004) and universal health coverage (Pearson r = 0.517, P = 0.0004).
-
●
AGE OF THE AFRICAN POPULATION
We next explored the role of demographic structure. The importance of age in COVID-19 mortality is well documented [13,[15], [16], [17]]. We observed a negative correlation between COVID-19 mortality and the percentage of the population under the age of 15 (Spearman r = −0.603; P = 4.3 E−06, Figure 3D). The percentage of the population over 65 was on the contrary positively correlated with COVID-19 mortality but to a much lesser extent (Spearman r = 0.436, P = 0.0017). This would explain why SSA has experienced fewer deaths than other continents with an overall population under 15 years of age that represents 48.2% for only 3.8% of inhabitants over 65 years of age. For example, Niger has the highest continental percentage of population under 15 (50.0%) and ranks 43rd for COVID-19 mortality in May 2021.
-
●
CO-MORBIDITIES
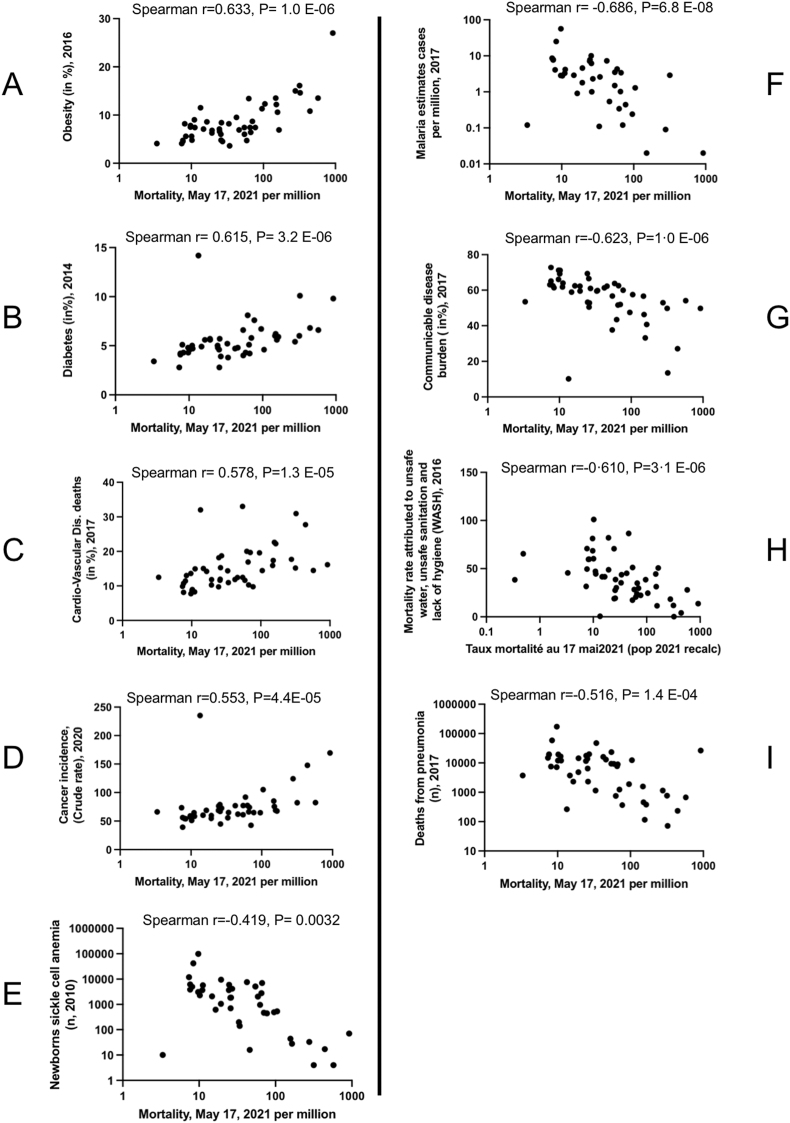
We then examined the impact of well-known comorbidities on COVID-19 mortality in SSA, as is routinely observed in high-income countries [12,13,[15], [16], [17]]. We searched for correlation between mortality and the most important non communicable diseases (medical data in Table 1, and Figure 4A–E). Obesity rate, diabetes, cardiovascular disease mortality and cancer incidence were positively correlated with COVID-19 mortality, with obesity rate being by far the most strongly correlated (Spearman R = 0.633, P = 1.0 E−06). On the contrary, the number of newborns with sickle cell anemia, the most frequent genetic defect in inter-tropical Africa, was inversely correlated with COVID-19 mortality (Figure 4E).
Figure 4.
Dot plots corresponding to the correlation between COVID-19 mortality and diseases. A-E Correlations with noncommunicable diseases, F–I Correlations with infectious diseases. Correlation of COVID-19 mortality rate in SSA A- Obesity rate (2016) in the adult population, B- Percentage of population with high blood glucose >7 mmol/l in 2014, C-Percentage of deaths related to cardiovascular diseases in 2017, D-Cancer incidence rate in 2020, E-Number of newborns with sickle cell disease in 2010, F-Number of malaria cases in 2017, G-Percentage of deaths from communicable diseases, H-Mortality rate attributed to unsafe water, unsafe sanitation and poor hygiene in 2016, I-Death rate from pneumonia in 2017.
SSA is part of the equato-tropical malaria endemic area [16,18]. COVID-19 mortality and malaria displayed a strong negative correlation (Spearman R = −0.69, P = 7.6E-08, Figure 4F). As already emphasized by others, this observation suggests a potentially protecting role of anti-malarial immunity or of anti-malarial prophylaxis on the severe forms of COVID-19.
Similarly, many features pertaining to infectious diseases such as communicable diseases burden, unsafe water-associated mortality, or death from pneumonia were negatively correlated with COVID-19 death rate (Figure 4G–I). Yet, all these conditions affect primarily children, and were significantly correlated with the proportion of the population under 15 while it was not the case for HIV infection or tuberculosis that concern mostly adult patients (see Supplemental Figure 1). As a consequence, although we cannot rule out that some of the above-mentioned conditions play to some extent a protective role against severe COVID-19, we favor the explanation that it is the proportion of children under 15 that plays a decisive role in the protection of the population against the disease.
-
●
ECONOMY AND DEVELOPMENT
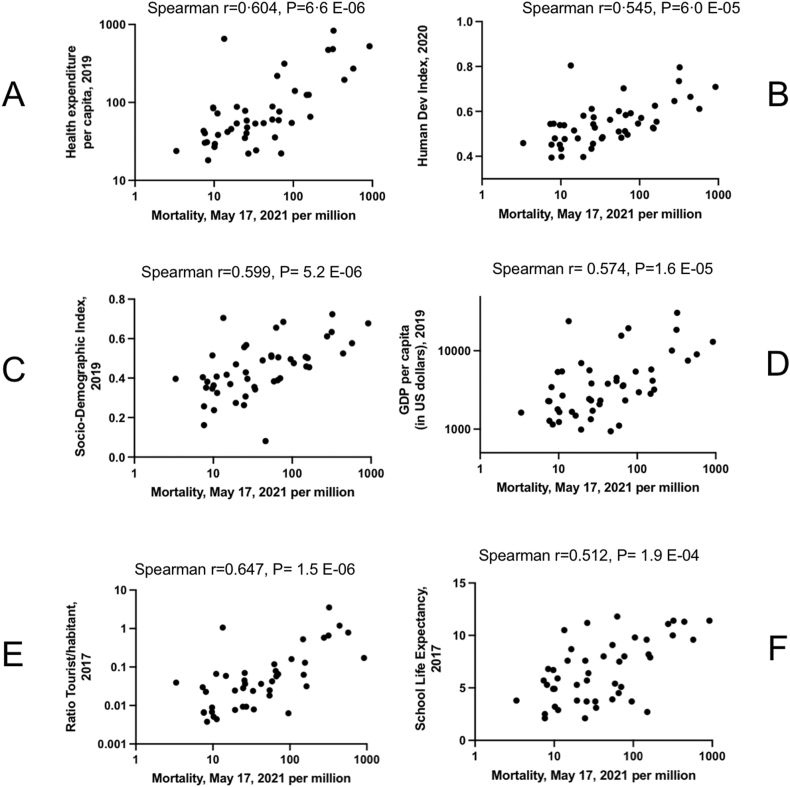
We tested thereafter the correlation between socioeconomic parameters and COVID-19 mortality (Figure 5A–F). The most highly correlated parameters were health expenditure per capita (Spearman r = 0.632, P = 1.84E-06), human development index (Spearman r = 0.617, P = 2.8E-06), socio-demographic index (Spearman r = 0.60, P = 5.2E-06), gross domestic product per capita (Spearman r = 0.574, P = 1.6E-05), average years of schooling (Spearman r = 0.572, P = 0.0001), and the ratio of tourists per capita (Spearman r = 0.647, P = 1.5E-06). Access to handwashing was not correlated with COVID-19 mortality (Supplemental Figure 2).
Figure 5.
Dot plots corresponding to the correlation between COVID-19 mortality and economic indicators. A-Health expenditure/capita, B-Human development index, C-School life expectancy, D-Socio-demographic index, E-GDP per capita and F-International tourism.
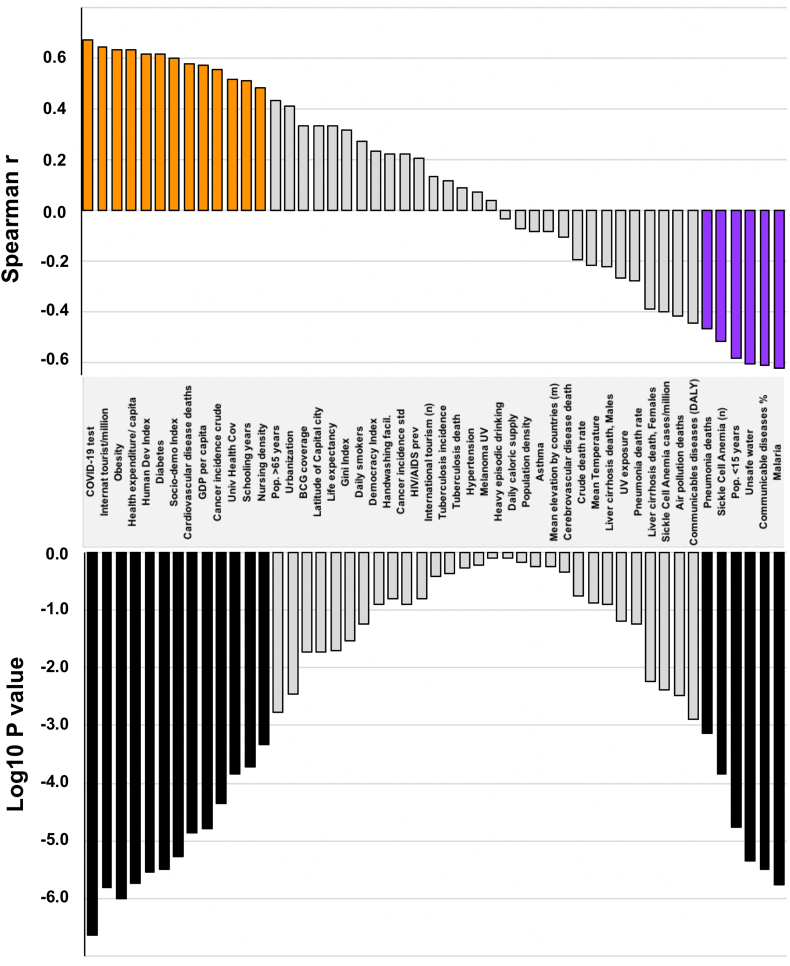
Figure 6 summarizes the different parameters studied with their Spearman coefficients and associated p-values.
-
●
MULTI-PARAMETRIC ASSESSMENT OF COVID-19 MORTALITY IN SSA
Figure 6.
Most important parameters influencing COVID-19 mortality and ranked according to their Spearman r coefficient. Associated p-values are displayed in the bar-chart below. Colored spearman coefficient bars correspond to parameters remaining significant after Bonferroni correction for multiple tests.
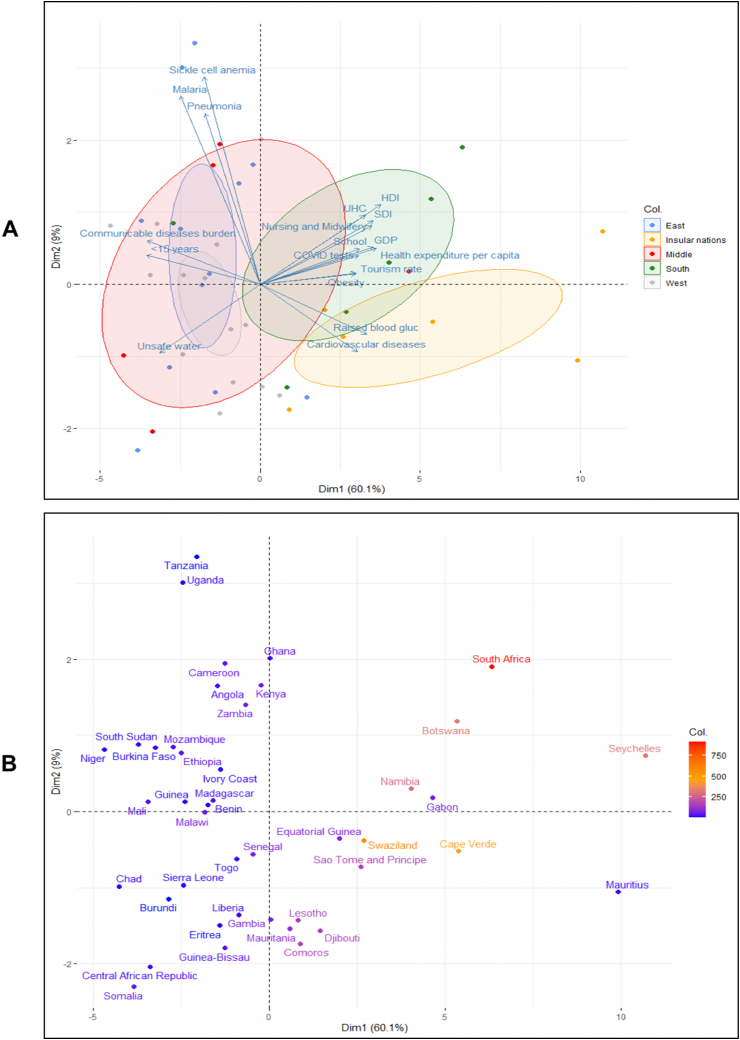
We next performed a principal component analysis (PCA) to figure out which parameters are the most influential for which countries. To this aim, we used the 19 features displaying the highest Spearman coefficients (Supplemental Table 2), and 45 SSA countries (we excluded the outliers Nigeria and Democratic Republic of the Congo, because of their high levels of malaria, pneumonia, and sickle cell anemia). We observed that countries in the Southern region and Island nations combine characteristics that set them apart from the rest of the continent (Figure 7A and B, Supplemental Table 1). The insular nations were more associated with non-communicable diseases (diabetes and cardiovascular disease-associated death) while nations from Southern Africa were more affected by obesity and economic indicators of affluence (HDI, GDP, Health expenditure, COVID-19 testing, etc). We confirmed these observations using Partial Least Square (PLS) and Orthogonal Partial Least Square (OPLS) analyses (Supplemental Figure 3A,B).
Figure 7.
PCA of parameters correlated with the deaths rates from COVID-19. The 1st dimension separates West, Middle and East regions from Southern and Insular nations. The 2d dimension separates Southern and Insular regions. A-PCA coloration according to geography. B-The same PCA with color code corresponding to the mortality rate.
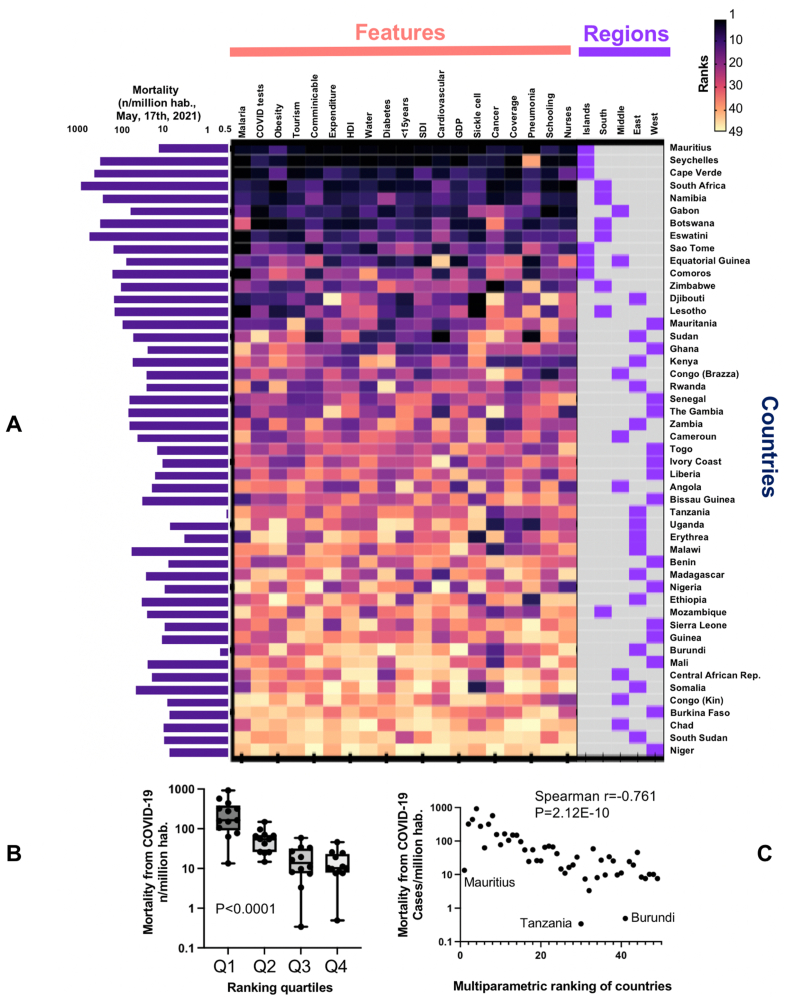
An analysis based on the risk ranking of each nation for these 19 most significant parameters yielded similar results (Figure 8A). Six southern countries (South Africa, Namibia, Botswana, Eswatini, Zimbabwe, and Lesotho) and five island nations on either side of the continent (Mauritius, Seychelles, Cape Verde, Sao Tome and Principe, and Equatorial Guinea, a country both continental and insular) rank among the nations at high risk for COVID-19 mortality, while four landlocked Sahelian countries (Chad, Burkina Faso, Niger, and South Sudan) forming a vast inland area along the southern edge of the Sahara appear comparatively protected from COVID-19. The synthetic risk combining the 19 parameters was significantly associated with the reported mortality from COVID-19 (Figure 8B and C). To assess the robustness of this approach, we analyzed our normalized data by hierarchical clustering. The results obtained were consistent with those obtained with PCA, PLS or ranking methods (Supplemental Figure 4).
Figure 8.
A-Matrix ranking SSA countries according to the most important parameters for COVID-19 mortality. Colors of the heatmap are given according to the rank of the country for each feature. B-COVID-19 mortality in SSA countries grouped in quartiles according to their synthetic score in the ranking. C-Dot correlating mortality and ranking in the matrix.
4. Discussion
Nearly two years after the start of the COVID-19 pandemic in December 2019, SSA countries have already experienced in the midst of 2021 several waves of contagion and deaths.
Several studies have shown that mortality is a more reliable indicator than morbidity; the number of SARS-CoV-2 infections is generally more underestimated than the number of COVID-19 deaths [12,13,17,19]. We thus focused on the latter. We observed a positive correlation between the number of PCR tests per capita and national rate of COVID-19 related deaths. This suggests that the intensity of testing reflects the strength of the epidemic and, indirectly, that the lack of proactive policies may contribute to underestimating the problem. However, countries that reported low population-based testing rates were among the lower-income countries of SSA and in these countries tests were plausibly targeted on severe cases only [20]. PCR testing including mildly symptomatic and asymptomatic people remains essential for a valid assessment of epidemiological indicators enabling anticipation of the next waves and implementation of adequate public health measures. A predictive study on data from 42 countries, published in April 2020, showed that a 10% increase in SARS-CoV-2 screening would result in an approximately 9% increase in new cases and a 9% reduction in case fatality [21]. Deliberate underreporting of cases at the government level may be part of the reason why mortality from COVID-19 is low in some SSA countries. The most extreme cases are Burundi and Tanzania. For example, in Tanzania, while the press reported an unusual number of deaths in some localities, the government claimed that the virus had been eradicated [22]. Tanzania reported only 509 infections and 21 deaths, all before June 2020. Symbolically, COVID-19 has been singled out as a potential cause of death both for the presidents of Burundi (Pierre Nkurunziza) and Tanzania (John Magufuli) in June 2020 and March 2021 respectively [23,24].
Since the screening policy is based on the importance of the population seeking health care at a given moment, it seems likely that the proportion of asymptomatic cases is high in SSA due to the tolerance of the disease by young people. A study based on the seroprevalence of SARS-CoV-2 infection in several African countries showed that almost 16.6% of the population of Kinshasa had been infected by autumn 2020, a rate that was almost 300 times higher than the official figures generated by positive tests. This rate is much higher than seroprevalence measured in Europe or India at the same period [25,26]. Extrapolating these figures to the entire population of the Congolese capital, nearly 2.4 million people would have been infected during the first wave of the epidemic between March and October 2020, contrasting with the 8,290 PCR-confirmed cases reported during the same period [27]. This seroprevalence survey shows that the virus may have circulated in Africa with an intensity similar to that observed in Europe and America.
COVID-19 mortality in SSA countries is affected by the demographic structure at both ends of the age pyramid [13,17,19]. Early reports from China highlighted aging as the most important risk factor for COVID-19 mortality, with case-fatality rates ranging from <0.5% for those under 50 to 3.8% between 60 and 80, and up to 14.8% for those over 80 [28]. Similar trends have been observed in the United States, with case fatality rates ranging from <1% for those under 50 to 10.4–27.3% for those over 85 [29]. Our analyses showed that in SSA, the impact of youth as a protective factor against COVID-19 mortality is greater than the role played by aging. Indeed, 40.2% of the population in SSA is under 15 while only 3.4% is over 65, compared to about 20% and 16% respectively in Europe and North America.
However, this youth advantage could have been potentially offset by other health burdens affecting African countries. With respect to comorbidities, we observed a positive correlation between COVID-19 mortality and the prevalence of obesity and diabetes, cancer incidence, and cardiovascular disease mortality. This would explain the high COVID-19 mortality observed in the southern part of SSA.
In addition to comorbidities, a striking observation was the strong inverse correlation that was found between malaria incidence and COVID-19 mortality. This confirms previous observations that reported low numbers of COVID-19 cases/deaths in populations submitted to endemic malaria [16,30,31]. Remarkably, geographic distributions of previous coronavirus diseases such as Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and SARS have indicated that regions with endemic Plasmodium infection have low incidence of these diseases [31,32]. Mechanisms explaining a similar negative correlation between endemic malaria and COVID-19 could be either due to: (i) the fact that natural anti-malarial immunity protects to some extent against the anti-SARS-CoV-2 immune overreaction [33]. (ii) Alternatively, as reported by Silas et al., this protection might be caused by immunodominant epitopes shared by SARS-CoV-2 and P. falciparum antigens. Indeed, several publications have noted the presence, on the N protein of SARS-CoV-2 of an apparently immunodominant epitope akin to thrombospondin-related anonymous protein (TRAP) of P. falciparum [30]. Others identified several shared tetrapeptide and pentapeptide epitopes between SARS-CoV-2 N or S proteins and P. falciparum TRAP or SSP-2 peptides [30,34]. (iii) Finally, studies have shown that commonly used antimalarial drugs display also some antiviral activity [30,[35], [36], [37]]. A recent study by Gendrot and colleagues observed that chloroquine, hydroxychloroquine, ferroquine, desethylamodiaquine, mefloquine, pyronaridine, and quinine inhibited SARS-CoV-2 viral growth in vitro in standard antimalarial treatment regimen [30,34,38]. Interestingly, according to the authors, these activities were superior to those of antivirals such as lopinavir, remdesivir or ritonavir currently under evaluation in clinical trials [30]. As in malaria endemic areas, a large subset of the population is regularly taking antimalarial drugs, it was hypothesized that this may contribute to the lower incidence of COVID-19 in these areas.
However, a major limitation of this study is the lack of standardization of screening and case reporting policies across countries. Like other regions, SSA countries underreport confirmed cases and deaths. In addition, the availability of individual patient data may be useful, but such studies are limited in African settings, and only a few individual factors have so far addressed frequency and outcome effects [39,40]. Furthermore, we should stress that our approach is only observational, and further studies are needed to address causality between correlated parameters and COVID-19 mortality. It is, at the moment indispensable to be very careful in interpreting our results. Indeed, one of the pitfalls inherent in this type of analysis is ecological fallacy which consists in giving a causal interpretation to a phenomenon only indirectly linked to what is being studied. In the present study, we suspect a series of pathologies to fall within this category as they affect more often or more severely children than adults (infectious diseases in general including malaria and pneumonia, and a genetic disease, sickle cell anemia). By the very fact of the protective nature of young age on COVID-19, all of them appear negatively correlated with mortality from this disease without playing any veritable protective role against it. Actually, the relative importance of these pathologies in each SSA country is also correlated with the proportion of the population under 15 years.
Our analysis shows that Southern SSA has a combination of demographic, climatic, and epidemiological disadvantages (Figure 7). Indeed, Southern African countries have the highest rates of the major comorbidities associated with mortality (cardiovascular, respiratory, and metabolic diseases) for COVID-19 [13,16,41,42]. South Africa, in particular, which has the highest COVID-19 mortality on the continent, has a higher prevalence of non-communicable diseases than any other SSA nation [13,43]. The positive correlation between mortality and parameters describing the level of development of populations appeared paradoxical at first sight. It confirms, however, the earlier observation that the most prosperous countries are the most affected by the COVID-19 [44]. This may be explained by the fact that increased life expectancy is associated with non-communicable diseases such as type 2 diabetes or obesity. School life expectancy, i.e. the average number of years spent in school, is strongly correlated with COVID-19 mortality suggesting that the intensity of schooling in a given country may contribute significantly to virus spread [12,45]. Schools as recognized hubs for SARS-CoV-2 circulation emphasizing that economic development may prove to be an Achilles heel rather than a protection against the virus [46]. Finally, high HDI populations are also very mobile and tend to attract tourists from abroad, increasing the risk of massive circulation of infectious agents. We observed, indeed, a very strong correlation between the number of tourists per million habitants and the mortality from COVID-19 in SSA.
The generalizability of our findings to other regions of the world is difficult to confirm insofar as the demographic situation of SSA is unique. Among the 50 countries with the largest proportion of the population under 15, 42 are located in SSA, making the population of this region collectively resistant to the deleterious consequences of SARS-CoV-2 infections. Emblematically, among the 14 SSA countries with less than 40% of the population under 15, five are island nations (only Sao Tome e Principe is not concerned) and five are nations located in its southern part (Zimbabwe and Mozambique are not present), the two geographical subsets most affected by mortality from COVID-19. Another aspect of our findings difficult to apply to the rest of the world concerns the other extremity of the human lifespan. Life expectancy is rather weakly positively correlated to COVID-19 mortality in our analysis (Spearman r = 0.332, P = 0.0195) albeit older age represents the most important risk factor of death in most of the northern hemisphere [47]. In SSA, it is instead comorbidities (obesity, diabetes, cardiovascular diseases, cancer) that occupy first ranks among the positively correlated risk factors of death. This situation is plausibly due to the almost universally small proportion of people above 65 in SSA (median proportion = 3.0%, IQR = 2.8%–3.6%) that inhibits the efficiency of correlation tests. Alternatively, the strong correlation of COVID-19 mortality with non-communicable diseases might be related to the low proportion of patients diagnosed and correctly treated for these diseases in Africa, making them perfect candidates for severe complications of COVID-19 [48].
5. Conclusion
Continued efforts to better understand the epidemiological dynamics in Africa seem essential, especially in the context of the third wave and the spread of Delta, Delta Plus, and Omicron variants. Despite significant progress, genome sequencing and monitoring of variants in SSA are not on par with other continents (to date, only 1% of genomes transmitted to the GISAID international surveillance mechanism come from Africa). Surveillance programs and local capacity building must improve to better understand the dynamics of future variants dissemination.
More than two years after the first case was reported in SSA, our study is one of the most comprehensive that tries to understand the peculiarities of the COVID-19 pandemic on this continent. Our study attempted modestly to assess the role played by different potential modulators of COVID-19 mortality in SSA. To do this, we have drawn on different sources and in different fields (demography, economy, epidemiology, climate) without seeking to limit or direct our conclusions in order to present them to professionals and the interested public without prejudice. Our analysis provides evidence for the role played by obesity, diabetes prevalence, cardiovascular diseases mortality, cancer incidence, tourism, age structure, and GDP (and development indices in general) in COVID-19 mortality in SSA.
Our data indicate that mortality from COVID-19 in SSA is apparently strongly influenced by multiple factors, both positively and negatively. Overall, the higher is the economic status of the country, the more at risk it is to face a high mortality at a national level. In this regard, the most important risk factor in SSA is, by far, the rate of obesity observed in the population, a feature tightly linked to wealth in SSA. While infectious diseases are known to preferentially afflict underserved populations living in low and middle incomes countries, COVID-19 stands as a new paradigm, representing the first infectious disease for which a fatal outcome is more frequent in the most affluent populations. In that sense, SARS-CoV-2 literally turned upside down the epidemiological paradigm that confines infectious problems to the poorest and non-communicable diseases to the wealthiest nations. Finally, it should be kept in mind that this work is based on correlations and that further works are necessary to demonstrate causal relationships.
Data sharing
The dataset analyzed for the manuscript is available upon reasonable request. The data dictionary is available on request to the corresponding author: pascal.pineau@pasteur.fr.
Declarations
Author contribution statement
Boris-Enock Zinsou; Diane Letourneur; Pascal Pineau, Ph. D: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Joël Siko; Raïssa Muriel de Souza; Frejus Adjagba: Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Erwan Sallard for proofreading the manuscript and Paul Clémençon for commissioning the R scripts that enabled us to produce the various maps of Africa.
Appendix A. Supplementary data
The following is the supplementary data related to this article.
References
- 1.Zhu N., et al. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019;7 doi: 10.1056/NEJMoa2001017. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathieu E., et al. Our World in Data; 2020. Coronavirus Pandemic (COVID-19) [Google Scholar]
- 3.Coronavirus disease (COVID-19) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 4.Mecenas P., Bastos R.T., da R.M., Vallinoto A.C.R., Normando D. Effects of temperature and humidity on the spread of COVID-19: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinaldi G., Paradisi M. An empirical estimate of the infection fatality rate of COVID-19 from the first Italian outbreak. medRxiv. 2020 2020.04.18.20070912. [Google Scholar]
- 6.Ghosh, D., Bernstein, J. A. & Mersha, T. B. COVID-19 pandemic: the African paradox. J Glob Health 10, 020348. [DOI] [PMC free article] [PubMed]
- 7.Azevedo M.J. 2017. The state of health system(s) in Africa: challenges and opportunities; pp. 1–73. (Historical Perspectives on the State of Health and Health Systems in Africa). Volume II. [Google Scholar]
- 8.Schardt C., Adams M.B., Owens T., Keitz S., Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inf. Decis. Making. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.GraphPad. https://www.graphpad.com/company/.
- 10.Salyer S.J., et al. The first and second waves of the COVID-19 pandemic in Africa: a cross-sectional study. Lancet. 2021;397:1265–1275. doi: 10.1016/S0140-6736(21)00632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei C., et al. Correlation of population mortality of COVID-19 and testing coverage: a comparison among 36 OECD countries. Epidemiol. Infect. 2020;149:e1. doi: 10.1017/S0950268820003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mboussou F., et al. Analysing the reported incidence of COVID-19 and factors associated in the world health organization african region as of 31 December 2020. Epidemiol. Infect. 2021;149:e256. doi: 10.1017/S095026882100193X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice B.L., et al. Variation in SARS-CoV-2 outbreaks across sub-Saharan Africa. Nat. Med. 2021;27:447–453. doi: 10.1038/s41591-021-01234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan J.R., Awan N., Islam M.M., Muurlink O. Healthcare capacity, health expenditure, and civil society as predictors of COVID-19 case fatalities: a global analysis. Front. Public Health. 2020;8:347. doi: 10.3389/fpubh.2020.00347. 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kircheis R., Schuster M., Planz O. COVID-19: mechanistic model of the african paradox supports the central role of the NF-κB pathway. Viruses. 2021;13:1887. doi: 10.3390/v13091887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Impouma B., et al. Estimating the SARS-CoV2 infections detection rate and cumulative incidence in the World Health Organization African Region 10 months into the pandemic. Epidemiol. Infect. 2021;149:e264. doi: 10.1017/S0950268821002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trends in global malaria burden. https://malariaatlas.org/trends/region/MAP/GLOBAL.
- 19.Pilecco F.B., et al. The effect of laboratory testing on COVID-19 monitoring indicators: an analysis of the 50 countries with the highest number of cases. Epidemiol. Serv. Saúde. 2021;30 doi: 10.1590/S1679-49742021000200002. [DOI] [PubMed] [Google Scholar]
- 20.Onyeaghala A.A., Olajide I. Managing COVID-19 outbreak in Nigeria: matters arising. Clin. Chem. Lab. Med. 2020;58:1645–1650. doi: 10.1515/cclm-2020-0748. [DOI] [PubMed] [Google Scholar]
- 21.Asahi K., Undurraga E.A., Wagner R. 2020. Benchmarking the CoVID-19 pandemic across countries and states in the U.S.A. under heterogeneous testing.https://www.medrxiv.org/content/10.1101/2020.05.01.20087882v1 05.01 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.John Magufuli: Tanzania’s president dies aged 61 after Covid rumours - BBC News. https://www.bbc.com/news/world-africa-56437852.
- 23.Burundi le président Pierre Nkurunziza est mort. TV5MONDE. 2020. https://information.tv5monde.com/afrique/burundi-le-president-pierre-nkurunziza-est-mort-gouvernement-362581
- 24.Magufuli John. BBC News; 2021. Tanzania’s President Dies Aged 61 after Covid Rumours. [Google Scholar]
- 25.Stringhini S., et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulenga L.B., et al. Prevalence of SARS-CoV-2 in six districts in Zambia in July, 2020: a cross-sectional cluster sample survey. Lancet Glob. Heal. 2021;9:e773–e781. doi: 10.1016/S2214-109X(21)00053-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nkuba A.N., et al. High prevalence of anti-SARS-CoV-2 antibodies after the first wave of COVID-19 in Kinshasa, Democratic Republic of the Congo: results of a cross-sectional household-based survey. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liuxingbingxue Zazhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Bialek S., et al. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) — United States, February 12–March 16, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osei S.A., Biney R.P., Anning A.S., Nortey L.N., Ghartey-Kwansah G. Low incidence of COVID-19 case severity and mortality in Africa; Could malaria co-infection provide the missing link? BMC Infect. Dis. 2022;22:78. doi: 10.1186/s12879-022-07064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rusmini M., Uva P., Amoroso A., Tolomeo M., Cavalli A. How Genetics Might Explain the Unusual Link Between Malaria and COVID-19. Front. Med. 2021;8 doi: 10.3389/fmed.2021.650231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masood N., Malik S.S., Raja M.N., Mubarik S., Yu C. Unraveling the Epidemiology, Geographical Distribution, and Genomic Evolution of Potentially Lethal Coronaviruses (SARS, MERS, and SARS CoV-2) Front. Cell. Infect. Microbiol. 2020;10:499. doi: 10.3389/fcimb.2020.00499. 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orish V.N., et al. Is malaria immunity a possible protection against severe symptoms and outcomes of COVID-19? Ghana Med. J. 2021;55:56–63. doi: 10.4314/gmj.v55i2s.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iesa M.A.M., et al. SARS-CoV-2 and Plasmodium falciparum common immunodominant regions may explain low COVID-19 incidence in the malaria-endemic belt. New Microb. New Inf. 2020;38 doi: 10.1016/j.nmni.2020.100817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oiknine-Djian E., et al. The Artemisinin Derivative Artemisone Is a Potent Inhibitor of Human Cytomegalovirus Replication. Antimicrob. Agents Chemother. 2018;62:e00288. doi: 10.1128/AAC.00288-18. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnard D.L., et al. Evaluation of Immunomodulators, Interferons and Known in Vitro SARS-CoV Inhibitors for Inhibition of SARS-Cov Replication in BALB/c Mice. Antivir. Chem. Chemother. 2006;17:275–284. doi: 10.1177/095632020601700505. [DOI] [PubMed] [Google Scholar]
- 37.Fan H.-H., et al. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus-related coronavirus model. Chin. Med. J. 2020;133:1051–1056. doi: 10.1097/CM9.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gendrot M., et al. Antimalarial drugs inhibit the replication of SARS-CoV-2: An in vitro evaluation. Trav. Med. Infect. Dis. 2020;37 doi: 10.1016/j.tmaid.2020.101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalal J., et al. COVID-19 mortality in women and men in sub-Saharan Africa: a cross-sectional study. BMJ Glob. Heal. 2021;6 doi: 10.1136/bmjgh-2021-007225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.James A., et al. An in-depth statistical analysis of the COVID-19 pandemic’s initial spread in the WHO African region. BMJ Glob. Heal. 2022;7 doi: 10.1136/bmjgh-2021-007295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.HIV and AIDS in East and Southern Africa | Avert. https://www.avert.org/professionals/hiv-around-world/sub-saharan-africa.
- 42.World Obesity Federation Global Obesity Observatory. World Obesity Federation Global Obesity Observatory https://data.worldobesity.org/.
- 43.Anjorin A.a., et al. Comorbidities and the COVID-19 pandemic dynamics in Africa. Trop. Med. Int. Health. 2021;26:2–13. doi: 10.1111/tmi.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khorram-Manesh, A. et al. Does the Prosperity of a Country Play a Role in COVID-19 Outcomes? Disaster Med. Pub. Healt. Prepp. 1–10. [DOI] [PMC free article] [PubMed]
- 45.Grant R., et al. Impact of SARS-CoV-2 Delta variant on incubation, transmission settings and vaccine effectiveness: Results from a nationwide case-control study in France. Lanc. Reg. Health. 2021:100278. doi: 10.1016/j.lanepe.2021.100278. Europe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meuris C., et al. Transmission of SARS-CoV-2 After COVID-19 Screening and Mitigation Measures for Primary School Children Attending School in Liège, Belgium. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.28757. e2128757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dessie Z.G., et al. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021:s12879. doi: 10.1186/s12879-021-06536-3. 021-06536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ataklte F., et al. Burden of undiagnosed hypertension in sub-saharan Africa: a systematic review and meta-analysis. Hypertension. 2015;65:291–298. doi: 10.1161/HYPERTENSIONAHA.114.04394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.