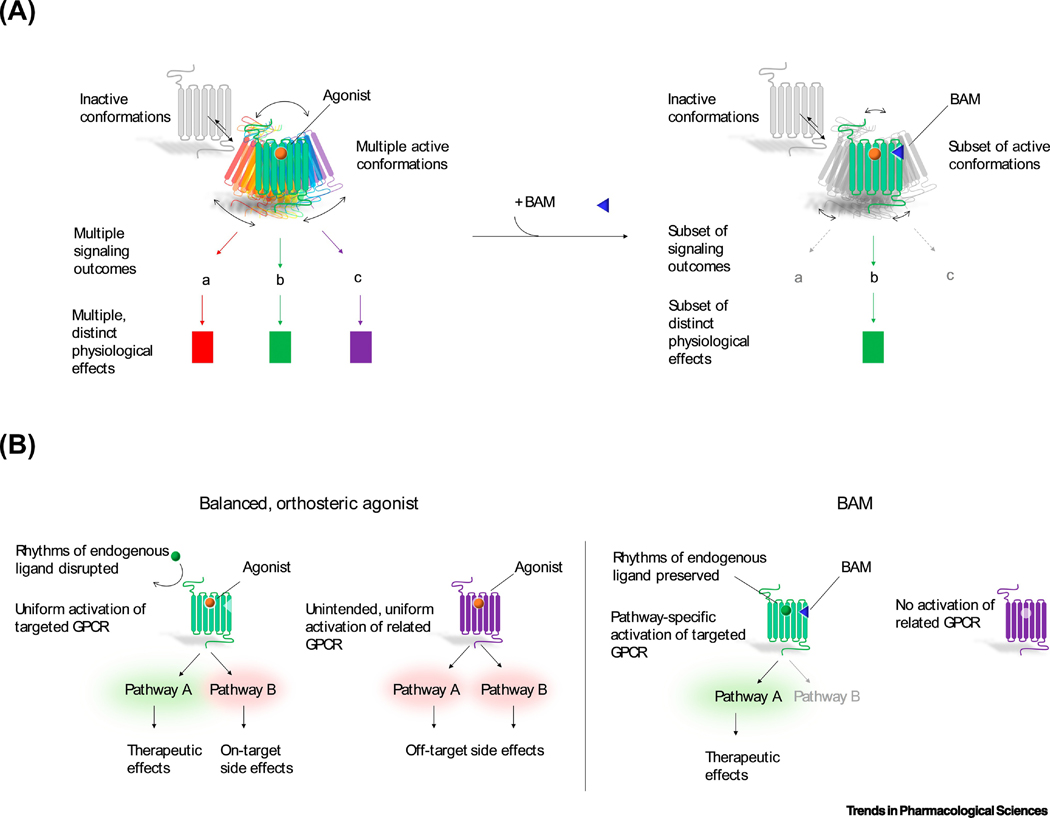
Figure 1, Key Figure. The molecular basis of biased allosteric modulation and its potential therapeutic advantages.
(A) Depiction of the molecular basis of biased allosteric modulation, based on the empirically supported conformational hypothesis of biased signaling. When a GPCR is activated by a balanced, orthosteric agonist (left), the receptor samples multiple active conformations, which permit receptor association with distinct effector proteins, including Gα family proteins (e.g., Gαs, Gαi/o, Gαq), G protein-receptor kinases and β-arrestins. These effectors give rise to multiple downstream signaling pathways (e.g., changes in cAMP levels, Ca2+ mobilization, ERK activation), which, in turn, lead to distinct cellular and physiological outcomes. Biased allosteric modulators (BAMs, right) confer more restricted pharmacological action. In the presence of a BAM, a GPCR samples only a subset of potential active conformations, leading to recruitment of only a subset of potential effectors. Consequently, signaling proceeds through a subset of potential pathways, producing a more limited repertoire of cellular and physiological effects. (B) Representation of the potential therapeutic advantages of BAMs. Balanced, orthosteric agonists (left) disrupt rhythms of endogenous ligands by blocking endogenous ligand binding and produce both on- and off-target side effects. By contrast, BAMs (right) do not prevent endogenous ligand binding, gain receptor subtype selectivity by binding to less well-conserved allosteric motifs and gain pathway selectivity by activating only a subset of signaling pathways.