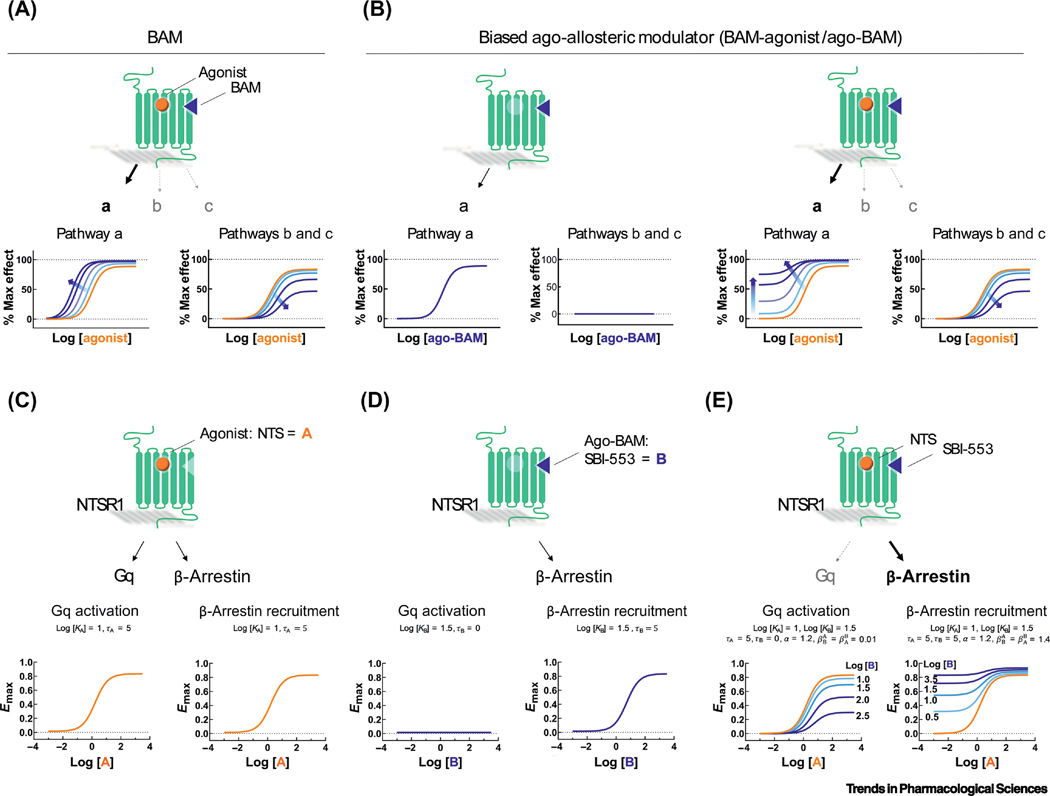
Figure 3. Effects of BAMs on receptor signaling.
GPCRs (green) are bound by an orthosteric agonist (gold ball) and an allosteric modulator (blue triangle). Binding of the agonist to the receptor results in signaling through multiple pathways - labeled by ‘a’, ‘b’ and ‘c’ (arrow thickness and pathway labels indicate the strength of the signaling outcome). Dose-response curves (below) show the effect of agonist on a given signaling outcome in the presence of increasing allosteric modulator concentrations, indicated by increasing blue color saturation. Newest among the classes of allosteric modulators are the BAM and ago-BAM. BAMs are pure modulators that lack intrinsic activity (τ=0) and may or may not alter the binding affinity (α) of the agonist. Characteristically, BAMs enhance signaling (β>1) through some pathways but exert no effect (β=1) or antagonize signaling (β<1) through other pathway subsets. Ago-BAMs act as biasing modulators to exert pathway-specific effects on agonist signaling and act alone as biased allosteric agonists, that is, τ is greater than 0 for some pathways, but not others. (A) This hypothetical BAM potentiates agonist-induced signaling through pathway ‘a’ while antagonizing agonist-induced signaling through pathways ‘b’ and ‘c’. (B) This hypothetical ago-BAM stimulates signaling through pathway ‘a’ when applied alone but has no effect on pathways ‘b’ or ‘c’. In combination with an orthosteric agonist, this ago-BAM enhances agonist signaling efficacy through pathway ‘a’ while antagonizing signaling through ‘b’ and ‘c’. (C-E) Application of Eq. 2, a symmetrical version of the extended operational model, to the agonist ago-BAM pair of neurotensin (NTS) and SBI-553 at the NTSR1. (C) NTS alone acts as a balanced, full orthosteric agonist, activating both Gαq- and β-arrestin-mediated cellular responses. (D) SBI-553 alone acts as a biased allosteric agonist, activating β-arrestin- but not Gαq-mediated cellular responses. (E) SBI-553 confers β-arrestin bias to NTS by concurrently enhancing NTS-induced β-arrestin recruitment and antagonizing NTS-induced Gαq activation. The qualitative behavior depicted here has been directly observed with the action on SBI-553 on NTSR1 [40, 41].