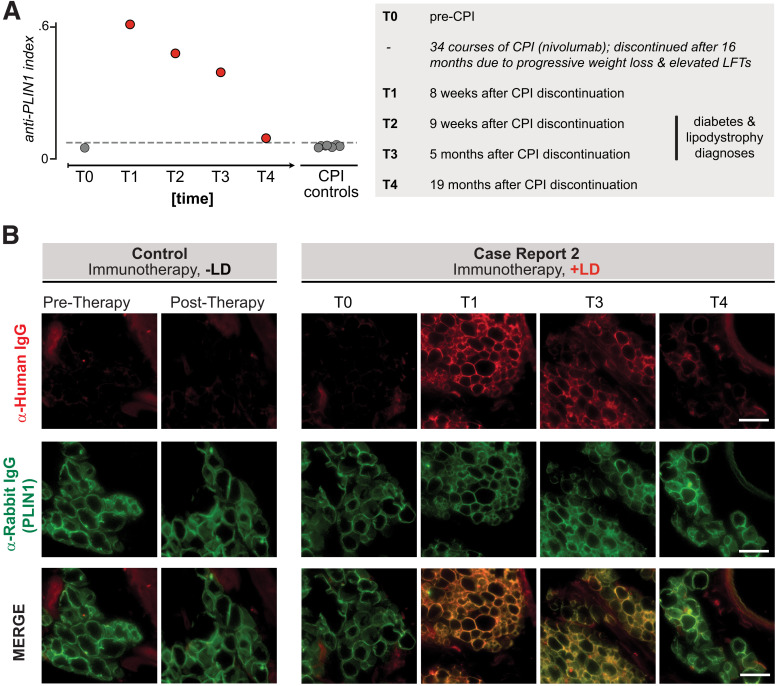
Figure 3.
Autoantibodies to PLIN1 in sera from a patient with autoimmune AGL following cancer immunotherapy. A: RLBA screening for PLIN1 antibodies in sera from all time points from index patient 2 as well as checkpoint-treated control patients without lipodystrophy (n = 7), as done for case report 1. Dotted line indicates mean ± 3 SD healthy control subjects (n = 11). Numbers to the right of circles indicate the time point series, for reference in panel B. The left panel describes the clinical timeline corresponding to time points T0–T4. Checkpoint inhibitor therapy (CPI) was discontinued at 34 cycles (16 months) due to progressive weight loss and elevated liver function tests (LFTs), with further workup over subsequent months. B: Validation of autoantibodies to PLIN1 with use of immunohistochemistry on mouse omental adipose tissue, as in Fig. 2B. Sera were used from either a control subject (immunotherapy but no AGL) or case report 2 patient (immunotherapy with AGL), with various time points. Each column represents an individual sample; from left to right: sera from checkpoint control pretreatment, sera from checkpoint control posttreatment with no autoimmunity, case report 1 pretherapy, case report 1 posttherapy 1, case report 1 posttherapy 3, case report 1 posttherapy 4. Images are 600 × 600 pixel insets from original ×40 image. Scale bar represents 100 μm. LD, lipodystrophy.