Abstract
The aim of this study was to perform a comprehensive literature review regarding the relevant hormonal and histological changes observed following Roux-en-Y Gastric bypass (RYGB). We aimed to describe the relevant hormonal (Glucagon Like Peptide-1 & 2[GLP-1 & GLP-2], Peptide YY [PYY], Bile Acids [BA], Cholecystokinin [CCK], Ghrelin, Glucagon, Gastric Inhibitory Polypeptide [GIP], and Amylin) profiles, as well as the histological (mucosal cellular) adaptations happening after patients undergo RYGB. Our review compiles the current evidence and further helps understand the rationale behind the food intake regulatory adaptations occurring after RYGB surgery. We identify gaps in literature where potential for future investigation and therapeutics may lie. We performed a comprehensive database search without language restrictions looking for RYGB bariatric surgery outcomes in patients with pre and postsurgical bloodwork hormonal profiling and/or gut mucosal biopsies. We gathered the relevant study results and described them in this review. Where human findings were lacking, we included animal model studies. The amalgamation of physiologic, metabolic, and cellular adaptations following RYGB are yet to be fully characterized. These constitute a fundamental aspect towards enhancing and individualizing obesity therapy.
Keywords: Metabolic adaptations, Roux-en-Y Gastric Bypass, Gastric Bypass, Bariatric Surgery, Obesity, Gut hormones, Satiety hormones, Mucosal adaptations
Introduction:
In 2017–2018, 42.4% of the adult population in the United States (~88.6 million) was reported to have obesity and 9.2% had severe obesity (~19.24 million)(1). It is estimated that by 2030, the prevalence of obesity in the US will reach up to 50%(2, 3). In 2018, the total annual medical costs attributable to obesity were estimated around $480.7 billion US dollars(4). More importantly, obesity is related to the development of obesity associated medical problems including coronary artery disease, type II diabetes, and end-stage renal disease, etc. Severe obesity increases the risk of these obesity associated medical problems even further(1), fostering additional expenses and compromising the population’s health and quality of life. Furthermore, obesity and severe obesity have been associated with increased all-cause mortality; hazard ratios ranging from 1.18 – 1.29 were found when all-cause mortality was compared between subjects with or without obesity(5).
Obesity is considered the result of an imbalance between energy intake and expenditure. The gut-brain axis plays a major role in regulating these factors(6, 7), where key factors including bile acids, and gut hormones interact with the hypothalamic brain centers (8–10) to regulate food intake. Following bariatric surgery, significant changes in bile acids and gut hormones have been described in patients with obesity when compared to their pre-surgical baseline (11, 12). In fact, alterations to levels of bile acids and gut hormones after metabolic surgery have recently been recognized as one of the mechanisms behind successful weight-loss outcomes(13).
Currently, numerous of bariatric surgical procedures are regularly preformed, with diverse outcome variability existing between the different operations, most likely due to differences in homeostatic adaptations following each procedure. Here, we review the adaptations following RYGB due to it having the best long-lasting consistent data and patient outcomes.
RYGB procedure description and evidence on basic variations: a small (<30ml) proximal gastric pouch consisting of gastric cardia is created by separating it from the remainder of the stomach. The small bowel is then divided at 50 to 100 centimeters distal to the ligament of Treitz. An alimentary “Roux-limb” (RL) is created by anastomosing the most distal segment of divided small bowel to the gastric pouch (gastro-jejunostomy). The “biliary limb” is then created by anastomosing the remaining segmented small bowel end to the jejunum (jejuno-jejunostomy) at a 75 to 150 centimeter distance from the gastro-jejunostomy. (Figure 1)
Figure 1:
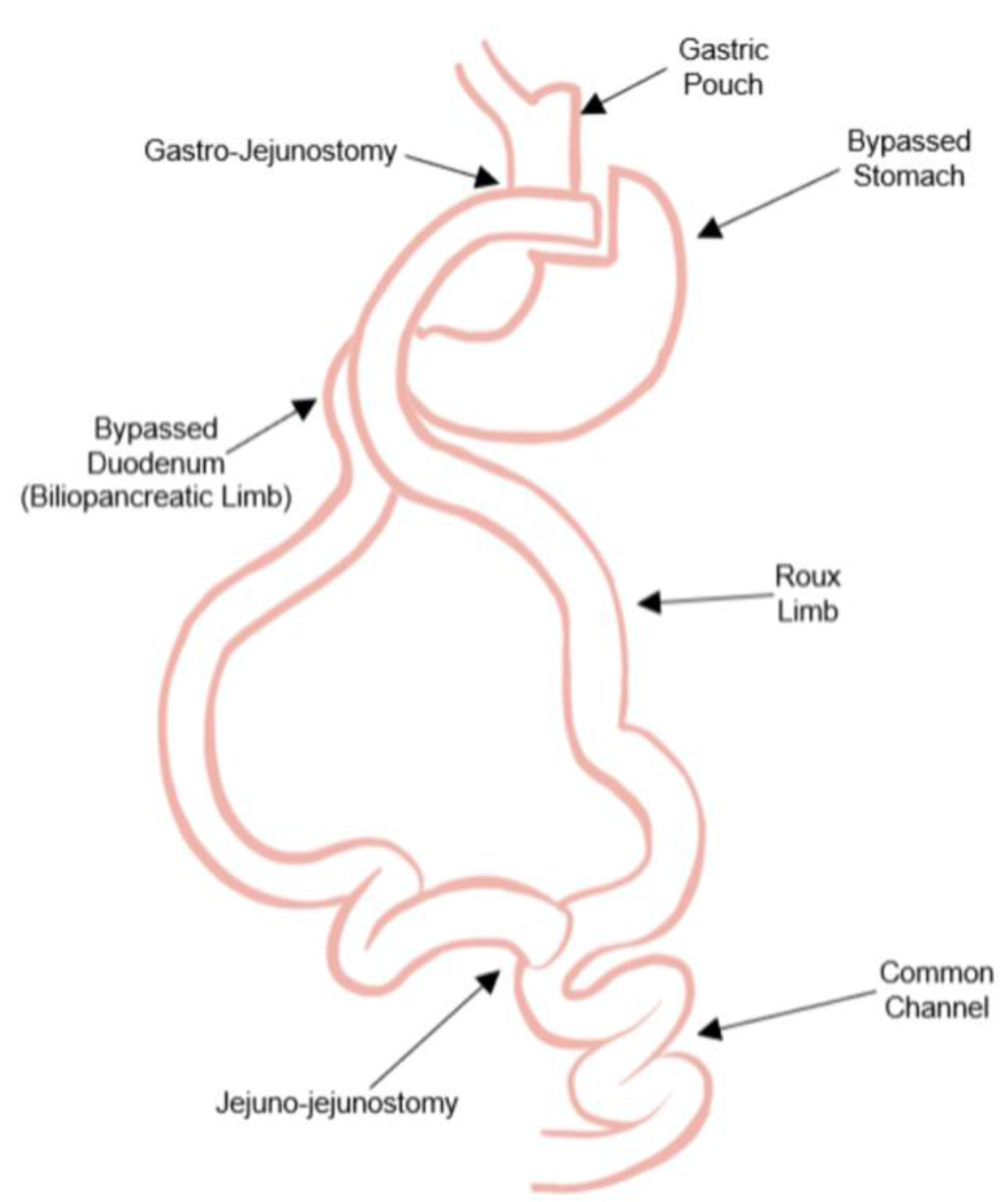
visual description of anatomical changes following RYGB.
The anatomical alterations to the gastrointestinal tract, surgically induced by RYGB surgery, promote homeostatic physiologic adaptations including reduced food intake, and altered gut hormone secretion, subsequently contributing to weight loss and weight loss maintenance (14). Variations in limb lengths have shown to effect surgical outcomes. Shorter common channels (CC) lead to higher chances of nutritional deficiencies with little to no effect on weight loss (15). On the other hand a longer biliopancreatic limb (BPL) seems to be related to better glycemic control and diabetes remission but not to nutritional complications (16). Furthermore longer BPL also seems to be related to better weight loss outcomes in patients with a BMI>50kg/m2 although evidence on the relationship between weight loss and BPL length is somewhat controversial (16). Total alimentary limb length (TALL) is not commonly measured or reported in RYGB studies. Some studies have shown correlation between shorter TALL and improved weight loss, however, several others have reported <400cm TALL with <200cm CC lead to protein malnutrition and possible limb lengthening procedures(17).
After RYGB, patients decrease their food intake following a series of changes in gut anatomy and physiology. We aimed to describe current evidence of cellular and hormonal adaptations following RYGB to provide a mechanistic explanation and rationale behind changes in food intake, sustained weight-loss outcomes, and to identify gaps in knowledge in the available literature. To the best of our knowledge, no previous reviews have focused on adaptations in a mucosal/cellular level and hormonal changes to establish possible associations and areas of interest for future research. We found that current evidence indicates that the increase in postprandial satiety hormones following RYGB is key to obtain appropriate weight loss outcomes. However, more information about enteroendocrine cell (EEC) adaptations, and gut hormone synergism during food intake regulation is needed. We did not perform a systematic review or meta-analysis. We also did not perform a search for specific mechanisms behind enhanced glycemic control as these were out of the scope of this review.
Methods:
We searched PubMed, Scopus, MEDLINE, Google Scholar databases (June 7, 2021, to Feb 1, 2022) with no language restrictions, for randomized clinical trials, case-control studies, case series, and case reports reporting bariatric surgery outcomes (Roux-En-Y Gastric Bypass [RYGB]), in patients with pre and postsurgical bloodwork profiling (GLP-1, GLP-2, PYY, CCK, Ghrelin, Oxyntomodulin [OXM], VIP) with or without gut mucosal biopsies. We used a range of terms including but not limited to “bariatric surgery, gastric bypass, Roux-en-Y gastric bypass, gut hormones, CCK, VIP, OXM, Oxyntomodulin, GLP, GLP-1, GLP-2, PYY, PYY3–36, Ghrelin, Bile acids, CDCA, taurocholic acid, conjugated bile acids, mucosal biopsies, histological adaptations, permeability, mucosal permeability, enteroendocrine, enteroendocrine cells”. We compiled the relevant findings in these studies and described them in this review. To further explain mechanism of action, we broadened our search and included studies with animal models where evidence in human experiments was lacking; human studies that aim to provide mechanistic explanations behind adaptations to bariatric and metabolic surgery usually have methodological limitations, and/or small sample sizes. Moreover, some of these studies assessed the outcomes of different bariatric interventions altogether. The results of these studies are widely variable due to methodological and aim variability.
Histological Adaptations:
The gut mucosa is the barrier and mediator between the gut luminal content and the basement membrane. There, paracrine, endocrine, and neuronal signals are sent following mucosal cellular stimuli and gut transit. EEC are directly stimulated by intraluminal gut content, which in turn induces gut hormone secretion. These hormones play an essential role in food intake regulation and digestion(18). Surgically induced changes in intraluminal content, and potential EEC adaptations could account for changes in gut hormone profiles following RYGB. We specifically searched for data on EEC changes, Chromogranin A, PYY and GLP mucosal staining, as well as postsurgical gastrointestinal tract biopsies.
Some studies have observed increased GLP-1 positive cells in the gastric fundus (19), and GLP-1 and Glucagon expression in the stomach, BP and AL(20). Apparently GCG (glucagon coding gene) increases in the BP and AL after RYGB. However, the significance of gastric GLP-1 expressing cells in the context of obesity is still uncertain, and the absence of a control group hinders the ability to compare these findings to other weight loss interventions. In humans, RYGB produces an increase in Ki67 (mitotic index) positive cells close to the bottom of the Lieberkuhn crypts of the AL(21), and a decrease of total mucosal surface (hypotrophy) (21, 22). Tight junction protein expression in the mucosal surface also changes possibly decreasing mucosal permeability(22). However, these findings are contrary to what has been observed in murine models. When compared with sham procedure, RYGB produces a noticeable alimentary limb mucosal hypertrophy greater than two-fold in the RYGB rats. RYGB doubles the total and regional L-cell numbers in murine models (23), and increases terminal ileum Ki67(24) when compared to sham procedures; these findings are yet to be evaluated in human models.
LITERATURE GAP:
Due to the discrepancies between human and animal physiology, it is often difficult to predict human physiology based solely on results from animal experiments. It is unclear whether the observed discrepancies are due to core physiological difference between species, location of said taken biopsies, or the postsurgical time when they were taken. Recognizing discrepancies and similarities in adaptations following bariatric surgery between species is important identify adaptations in variables (those that are preserved between species) as a consequence of RYGB. It is important to describe these changes in EEC specially in human models, to characterize the surgically induced satiety hormone secretion pathway and find new ways to enhance current obesity therapies.
Hormonal Adaptations
GI satiety hormones
GI satiety hormones GLP, PYY, and OXM are secreted by L-type EECs (L cells) and function to reduce food intake through their action as anorexigenic signals at hypothalamic arcuate and paraventricular nuclei as well as by altering gut motility through the ileal break mechanism. Previous literature has established the elevation of these L cell secreted hormones in circulation (25) in patients after undergoing RYGB (26). Sole infusion of these hormones (GLP-1, PYY, and OXM) grants inferior weight loss than RYGB (27) suggesting that these outcomes are not solely due to elevated hormone levels. Factors like speed and degree to which hormones peak, associated to the anatomical alterations and other adaptations surely impact weight loss.
Following RYGB surgery GI physiology is also altered. Gastric pouch emptying findings are controversial, some studies show faster gastric emptying for liquids and slower gastric emptying for solids(28–30). Others show overall fast pouch nutrient emptying into the jejunum being related to elevated postprandial gut hormones (31). These discrepancies might be due to differences in gastro-jejunal anastomosis (GJ) diameter, and/or pouch size. They could also be due to differences in pouch emptying measurement techniques following RYGB(32). While there is a trend and an association(33), GJ diameter and pouch size do not in and of themselves seem to be causal agents of poor weight loss responses(32, 34, 35). More robust longitudinal studies comparing postop pouch size, GJ diameter, and pouch emptying, with long term follow up measurements of these same variables (under standardized techniques) are needed to evaluate and clarify the impact of these on weight loss outcomes, weight regain, and hormonal and metabolic adaptations following RYGB.
Despite the changes in gastrointestinal motility being variable, RYGB consistently induces postprandial elevation of satiety hormones, increased satiety (mainly through the arcuate and paraventricular nuclei of the hypothalamus), thus, causing early postprandial satiety and/or reducing hunger.
GLP-1:
GLP-1 is secreted by intestinal L cells in a biphasic pattern (15–30 & 90–120 minutes postprandial) when stimulated by luminal contents. Protein, fat, and glucose (36) are strong GLP-1 stimulating agents, as shown by their direct administration of these into highly perfused intestinal lumen (37–39). Aside from its commonly known incretin effect, GLP-1 acts upon food intake regulation via central and peripheral effects (40). Intravenous infusions of GLP-1 significantly decrease food intake in lean subjects, subjects with obesity, and with type 2 diabetes mellitus in a dose dependent fashion (41–44).
Following RYGB, fasting plasma GLP-1 has been shown to be either normal, or elevated in human models (24, 45). Human postprandial GLP-1 levels have been shown to be elevated up to 40 months after RYGB when compared to pre-surgical measurements (24, 26, 45–56). A recent meta-analysis showed that peak postprandial GLP-1, 30 minutes after a meal is the most consistent elevated measurement after RYGB(45). Furthermore, GLP-1 has also been shown to be significantly elevated in RYGB in comparison to other weight-loss interventions including diet and adjustable gastric banding.
Direct delivery of nutrients into the proximal jejunum is associated to these differences in postprandial GLP-1 levels. Postprandial GLP-1 reaches higher levels when administered through an oral route in patients who had RYGB, when compared to a gastrostomy route into the gastric remanent (57). Some murine models have shown similar findings and reversal of the increased postprandial GLP-1 detection with nutrient administration through a gastrostomy route (58–60). This route dependent difference in GLP-1 secretion has been suggested to be the result of a rapid gastric-pouch emptying of nutrients into the Roux limb (11). This might not be the only mechanism by which GLP-1 elevates following RYGB, as other bariatric procedures also increase the speed of nutrient delivery into the small bowel but don’t display the same increase in postprandial GLP-1 or weight loss and metabolic outcomes (61).
Following RYGB, human studies have shown that an attenuated increase of postprandial Peptide YY (PYY) and GLP-1 secretion is associated to poor weight-loss response or maintenance (14). Animal models have demonstrated how responsiveness to GLP-1 receptor agonists could predict some metabolic benefits secondary to RYGB (62). Somatostatin secretion from D-type EECs inhibits GLP-1 and PYY3–36 secretion. Administration of somatostatin analogues in RYGB patients has shown to decrease fullness ratings and increase meal size(14), while reduced hunger and early satiety are associated with elevated satiety hormones.
Orexigenic nuclei and the insula have decreased activation with visual food stimuli and food ingestion respectively in patients with a history of RYGB (63). In humans, inhibition of GLP-1 receptors with Exendin 9–39 after RYGB increases activation of orexigenic nuclei with visual stimuli, as well as increased activation of the insula following food intake(63). Weight loss outcomes and maintenance are likely the result of varied food intake regulatory mechanisms. Increased satiety hormone secretion seems to be fundamental towards obtaining optimal weight loss outcomes.
GLP-2:
Glucagon-like peptide 2 (GLP-2) delays gastric motility, induces cellular proliferation, regulates apoptosis, intestinal nutrient absorption, and permeability in the gastrointestinal tract(64). Among hormonal adaptations, an overall increase of GLP-2 has been suggested to occur following RYGB. Animal models have shown a two-fold increase in immunohistochemically marked GLP-2-possitive cells in the alimentary limb (23) as well as increased plasmatic fasting GLP-2 (24) after RYGB. Despite these results, discrepancies between animal studies’ results and human physiology represent a major barrier. Human experiments have demonstrated no significant differences in postoperative fasting levels of GLP-2 with when compared to pre-surgical levels(24). Postprandial levels on the other hand have been shown to be elevated significantly as early as 2–6 weeks after surgery and persist elevated for up to 12 months (24, 65, 66).
Postprandial increased GLP-2 levels could be related to the observed decrease in intestinal permeability. However, these GLP-2 findings do not correlate with the hypotrophic mucosal changes described previously. There is still much to learn on how GLP-2 adaptations following RYGB affect gut physiology and weight-loss outcomes.
PYY:
PYY is secreted by gut L-type EECs; it slows gastric emptying through the ileal break mechanism (i.e. slowing of gastric emptying secondary to ileal EEC secretion of PYY), increases postprandial satiety, and regulates energy expenditure (67). Food intake regulating actions elicited from PYY have been shown to be primarily mediated by PYY3–36 (68, 69). Several murine based studies have shown PYY’s effect upon the Central Nervous System (CNS). Through injection of Neuropeptide y 2 Receptor (NPY2R) agonists and antagonists into the CNS as well as peripherally, PYY3–36’s central and peripheral effect reducing food intake has been showcased (70–72). Even stimulation of tongue NPY2R receptors with PYY3–36 has shown reduced food intake in murine models (73). Peripherally injected PYY3–36 in human experiments corroborates these findings. Supraphysiological doses produce reduced meal sizes, whilst lower doses have only displayed increased fullness (68, 74). It is possible that its functions upon food intake regulation, are potentiated due to synergism with other gut hormones like GLP-1.
Similarly to GLP-1 the nutrient route (oral vs. gastrostomy) after RYGB seems to be intimately related to evaluated postprandial PYY secretion (57). Postprandial plasma levels of PYY also rises in patients following RYGB (14, 46, 47, 75, 76). More so, studies have described a potential relationship between postprandial PYY with good and poor weight loss responders, where higher levels of postprandial PYY were associated to good responders (14, 77).
Despite these reports, there has been a lack of consistency regarding reported postprandial PYY curve patterns, and Areas Under the Curve (AUCs) after RYGB surgery (26, 48). Further studies with standardized blood sample timing-recollection techniques, meals, and PYY3–36 measuring techniques are needed to comprehensively describe this gut hormone’s pre and postprandial pattern after RYGB.
OXM:
This satiety hormone is derived from proglucagon and is secreted alongside GLP-1 following food intake. OXM influences energy balance by reducing hunger and energy intake while increasing energy expenditure. It stimulates GLP-1 and glucagon receptors thus regulating gastric motility and secretion, energy balance, and glucose homeostasis(78, 79). Subcutaneous administration of OXM induces weight loss in humans (80, 81).
Following RYGB patients display elevated postprandial OXM(82). Overall resting energy expenditure is decreased, but when analyzed from an objective perspective correcting for anthropometrics or body composition it has been shown to display more efficient pattern (83) as well as a relationship to weight loss, both of which might be related to the increase in OXM. Postprandial OXM levels have been described as a predictor of weight loss after RYGB; when associated to GLP-1, PYY, Glicentin, and Ghrelin they predict 60% of the variations in weight loss and 19% of the variations in food intake (84). These findings highlight the importance of enhanced satiety hormone secretion for weight loss outcomes after RYGB.
Bile Acids:
BA are considered important mediators of food intake regulation. Conjugated BA have been identified as secretagogues inducing GLP1- and PYY secretion by EEC (85).
Bile acid pathway:
95%(86, 87) of BA reuptake happens through the apical Na+-dependent bile salt transporter (ASBT) in the distal ileum (88). Remaining luminal BA undergo deconjugation and dehydroxylation by colonic bacteria. Passive colonic reabsorption of BA recovers some of the remaining bile salts (5%) (89). At this level, reabsorbed BAs act as endogenous ligands for farnesoid X receptors (FXR) and G-protein-coupled bile acid receptor 1 or Takeda G-protein-coupled receptor 5 (GPBAR1 or TGR5) in the enterocyte (86). These two receptors are stimulated intracellularly, inducing production of fibroblast growth factor 19 (FGF-19) and GLP-1, respectively. There are several other bile acid receptors that are less well studied but could very well be important mediators of these same effects.
Decreased FGF-19 levels are present in patients with obesity and diabetes (85). FGF-19 (equivalent to FGF-15 in rodents) generates negative feedback into the hepatocytes suppressing CYP7A1 in human hepatocytes and repressing BA biosynthesis(90)(91). FGF-19 is related to increased mitochondrial activity, protein synthesis, and decreased adiposity (90). Three months after RYGB, FXR induced FGF-19 has shown to be elevated in human models(92). FGF-19 has also been showed to be correlated to increased BA, PYY, and the incretin effect (93, 94).
TGR5 has been observed in human colonic mucosal biopsies that were also GLP-1 positive (85). After ex-vivo stimulation with taurocholic acid, colonocytes have shown increased GLP-1 and PYY mRNA expression as well as GLP-1 release(85). Stimulation of TGR5 results in a rapid increase of these gut hormones, reduced appetite, and improvement in insulin sensitivity (95, 96). (Figure 2)
Figure 2:
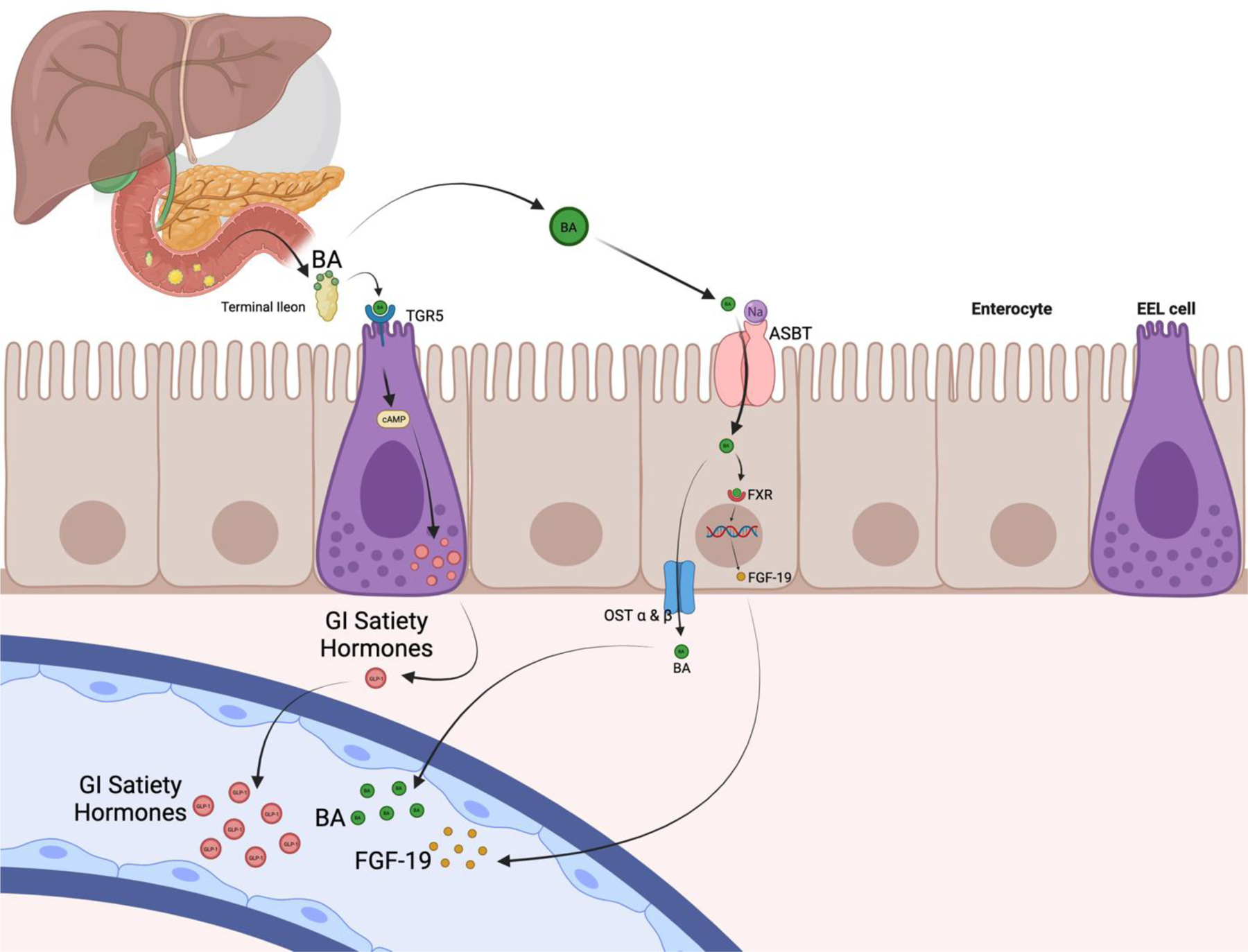
Bile Acids and satiety hormones pathway graphic description. Created with BioRender.com
BA & RYGB:
Ahmad et al. saw that the prandial and postprandial BA levels in patients with obesity were decreased when compared to lean, and that the prandial and postprandial rise in BA was restored by RYGB in humans(93, 97). After RYGB surgery in humans, total(12), fasting(98), prandial and postprandial plasmatic BA levels increase significantly and stay elevated for years (99), while stool bile acids decrease. Colonocytes express BA signaling machinery directly linked to gut hormone secretion (85). Patients with obesity and diabetes have showed a deficiency of this machinery (85). Comparisons with other weight loss interventions(98) suggest that the elevation in serum bile acids could possibly be independent to weight loss. Multiple animal models have been employed to try and explain the changes in BA metabolism following bariatric surgery. Bile diversion procedures in diet induced obesity mice models showed that direct bile delivery into the terminal ileum in the absence of stomach or intestinal remodeling, leads to weight loss, fat malabsorption, and improved glucose tolerance(100). The same bile diversion procedure increases incretin tone with no weight loss effect on lean mice with low-fat diet; thus, suggesting BA involvement in food intake and satiety gut hormone homeostasis. These observations following bile diversion, are inhibited by the administration of exendin-9 (GLP-1 Receptor antagonist) as well as in GLP-1 receptor knockout mice, and in TGR5 intact but FXR null mice. These findings reveal the importance of the FXR signaling towards the established incretin effect, whilst showcasing BA mediation of clinically important metabolic outcomes seen following RYGB.
Human and murine models have shown that the BA adaptations following RYGB are not immediate and are probably a consequence of the metabolic adaptations of the surgery(101, 102). The increase in plasmatic BA levels seems to be secondary to surgically induced BA exposure to the small bowel and probably not due to increased liver production, or increased enterohepatic circulation (102).
Secondly, TGR5 (GBPAR1) and BA have both been identified in astrocytes and neurons of the human brain, suggesting involvement in CNS function(103). Animal models have identified BA receptors to addiction centers and key reward signaling centers while establishing their role as hormonal mediators regulating the dopaminergic reward pathway (104). These results are supported by translational studies revealing attenuated alcohol consumption following RYGB in humans and rats which has been correlated to GLP-1 secretion (105). Additional studies are needed to further elucidate BA’s function (if any) in CNS food intake regulation.
It is yet to be determined if changes in BA impact food intake directly or through hormone mediators. Studies evaluating correlation, and causality are needed to assess the degree of influence BA have on “in vivo” gut hormone secretion, glycemic control, and metabolic markers. Randomized clinical trials exploring its potential ability to work as a therapeutic adjuvant in obesity therapy could provide the very much needed evidence.
Cholecystokinin (CCK):
CCK induces contraction of the gallbladder, relaxation of the sphincter of Oddi, and inhibition of gastric emptying by relaxing the gastric fundus(106). CCK has an important neuroendocrine role in food intake regulation. In human models, intravenous CCK infusions at physiological concentrations have shown to reduce meal size (107, 108). Furthermore, intravenous infusions of CCK receptor-A (CCKAR) antagonists increase meal size, pre-meal hunger, and reduces fullness (109). In vagotomized rat models, infusion of a CCKAR antagonists that crossed the blood brain barrier decreased food intake in contrast of immunoglobulin bound CCKAR antagonists (can’t cross the blood brain barrier) which didn’t decrease food intake(110, 111).
The vast majority of CCK producing cells remain in the bypassed duodenum and proximal jejunum after RYGB surgery. Despite the anatomical modification, normal (112, 113) or elevated postprandial CCK plasma levels have been shown as early as 1 week and up to a year after surgery in human models (26, 66). Interestingly, the most pronounced elevation of CCK was showed at 3 months and up to a year postop., speculated to be secondary to CCK secreting cell proliferation (26).
Ghrelin:
Ghrelin is an orexigenic hormone who inhibits insulin secretion, decreases energy expenditure, lipolysis, and increases adipogenesis among many other functions (i.e., bone metabolism, muscle cell differentiation, oncogenesis)(114). The highest density of ghrelin secreting cells is located in the stomach; however, ghrelin secreting cells have also been identified in the duodenum, ileum, cecum and colon.
Patients with obesity may have elevated fasting ghrelin plasma levels(26). Evidence suggests an inhibited postprandial plasmatic ghrelin drop in patients with obesity when compared to lean subjects (26, 115, 116). Following RYGB, fasting and postprandial ghrelin plasma levels have been shown to be reduced immediately after surgery(26, 47, 117–119). It’s important to note that there has been significant difficulty reproducing the results from Cummings, et al. 2002 NEJM(117). Studies with greater follow-up longevity show more controversy in plasmatic ghrelin values following gastric bypass (120), It is clear that following RYGB, there might be some acute decreases in ghrelin; but there have also been reports of studies where ghrelin levels return to, or approach baseline years after RYGB. Despite returning to elevated fasting levels, some patients who undergo RYGB seem to regain the previously absent postprandial ghrelin drop (26). A recent meta-analysis showed overall short-term decrease and long-term increase in fasting ghrelin following RYGB (121). Furthermore, weight regain has been associated with increased pre and postoperative ghrelin levels(122).
LITERATURE GAP:
Compiling and comparing study results characterizing changes in the hormonal profile following RYGB is limited by differences in methodological measurements of each hormone, and the actual panel hormones being observed (usually secondary to what each investigating team considered relevant). Ghrelin results in particular have had a historical difficulty in being reproduced due to high variability in the commercially available assays(123, 124). Subsequently no study simultaneously assesses through time the amalgamation of hormonal and histological adaptations that synergically change food intake regulating signals after RYGB. A thorough mechanistic explanation supporting why hormonal profiles change, particularly why satiety hormones rise following RYGB surgery is yet to be described. Total PYY increases have been described but there is still uncertainty about the curve or secretion pattern due to contradictory evidence. There is a vast knowledge gap regarding the determination of bile acids as direct or indirect mediators of food intake regulation. Finally, a pathway that differentiates post RYGB adaptations from the ones happening in every other weight loss intervention, hasn’t been fully characterized.
Other GI hormones:
Due to the results of current anti-obesity medication trials(125–127), we decided to investigate other hormones that might be related to the outstanding results following RYGB. This was done with the purpose of further elucidating the hormonal adaptations and their relationship to weight loss outcomes.
Glucagon:
Glucagon, classically classified as a contra-regulatory hormone is secreted from the pancreatic α-cells in response to low blood glucose. Glucagon has a plethora of functions throughout human physiology. It is most commonly known for promoting liver gluconeogenesis and glycogenolysis (128), but glucagon is also involved in energy balance regulation (food intake and energy expenditure) (129, 130), lipid metabolism(131), and insulin secretion(132). Glucagon levels increase postprandially after food ingestion in humans (133, 134). Animal models have displayed increased food intake with antibody mediated glucagon inhibition (135) and decreased food intake with GLP-1/Glucagon dual stimulation (136, 137).. These findings point towards the importance of glucagon in food intake regulation.
Following RYGB there is a continuous increase in glucagon up to 6 months after surgery(138). Postprandial glucagon secretion also seems to be increased following RYGB(139, 140). Despite of this increase in secretion, there seems to be some degree of secretion dysregulation evidenced by the lack of difference in postprandial glucagon secretion between RYGB postoperative patients with and without hypoglycemia(139, 141). When comparing before and after RYGB, there are reports of increased GCG (glucagon coding gene) expression from BPL and RL biopsies 3 months after RYGB (20). It’s important to mention that this study did not have a control group to differentiate from other weight loss interventions. Further studies are needed to accurately characterize the GCG gene expression throughout the GI tract following RYGB.
Gastric Inhibitory Polypeptide (GIP):
GIP is one of the first incretin hormones to be described, GIP stimulates insulin secretion in healthy patients and patients with diabetes (142–144). Furthermore, animal models, and cell experiments have shown that GIP stimulates lipogenesis(145–147), and beta cell proliferation(148, 149). More recently, novel animal models have identified GIP receptors in energy regulating CNS related centers(150), and that high central doses (151) and acute intra-cerebroventricular (152) doses of GIP lead to decreased food intake and induced weight loss which is blunted in GIPR knockout mice.
In general, evidence suggests that fasting and postprandial GIP levels tend to decrease following RYGB(153); this phenomenon is particularly marked in patients with diabetes. Weight loss, pouch volume, BPL and RL length, don’t seem to be significant predictors of the decrease in GIP following RYGB.
Amylin:
Amylin is a hormone primarily expressed and produced by the β pancreatic cells. Its secretion is stimulated by glucose, amino acids, and fatty acids(154–156). Amylin is co-secreted along with insulin, incretins like GLP-1 stimulate its release (157). Multiple animal and human models have studied the function of this hormone and described its role as a signal of satiation (158). In rats, it has been shown to control meal size (159, 160) thus acting as a satiation signal at the level of the area postrema (161) as well as other energy regulating brain centers. Human studies have shown fasting(162) and postprandial amylin to be elevated in obesity(163–165).
In patients without diabetes, two and four weeks after RYGB postprandial amylin levels don’t seem to display any significant change (166, 167). Despite this, in patients with diabetes there seems to be a decrease in amylin one year after RYGB (168, 169). Contrast to what is seen in humans, mice models demonstrate increased postprandial amylin secretion following RYGB(170).
Amylin seems to play a significant role in food intake regulation as a satiation signaling hormone. It is not clear weather amylin levels are increased in obesity due to the general increase in food intake, or due to signaling pathway dysregulation (or both). Evidence on the effects of RYGB on amylin secretion is lacking, it is not clear whether the changes in amylin are due to weight loss, time, or having pre-existing diabetes. Furthermore, the inter-species discrepancies in amylin adaptations following RYGB are not clearly elucidated either.
Limitations:
The nature of this literature review doesn’t permit a statistical comparison between study results which poses limitations towards drawing conclusions or compiling data. Comparing results is also limited by methodological differences between studies. For example, a lack of standardized post-prandial time points for bloodwork measurements are limitations for comparisons between gut hormone levels. Finally, this is not a systematic review and therefore it is subject to bias from our points of view as writers and gaps in literature.
Conclusion:
Current human studies show mucosal hypotrophy and decreased permeability of the alimentary limb following RYGB. Mucosal changes after RYGB at a cellular level regarding morphology, EEC count and function (mRNA and/or protein expression) are yet to be appropriately described.
GLP-1 and GLP-2 plasmatic levels have been shown to be postprandially elevated following RYGB. Their close relationship to gastric motility and food intake regulation (GLP-1) are most likely to be implicated with the sustained weight-loss effects of RYGB. Postprandial levels of PYY are elevated after RYGB. Accurate characterization of postprandial PYY, particularly PYY3–36 curves after RYGB surgery is yet to be demonstrated. There is a correlation between degree of gut hormone (GLP-1 and PYY) increase and successful weight loss outcomes following RYGB. BA levels have been shown to be more than two-fold significantly elevated after RYGB surgery. Their level of influence on gut hormone secretion, and food intake regulation in humans is yet to be described. Evidence from animal models suggest pivotal importance of BA as mediators of satiety gut hormone secretion following RYGB, and the neuronal reward seeking behavioral pathway. Post-prandial plasmatic CCK levels are also increased following RYGB despite the majority of CCK secreting cells being isolated from direct nutrient stimuli. This could be due to CCK secreting cell proliferation. Studies describing this change in CCK-secreting cell population in humans, and its synergistic effect on food intake regulation could birth new hypothesis about its role and possible therapeutic implications in weight loss therapies. Plasmatic ghrelin levels after RYGB have shown variable results. Restoring the post-prandial ghrelin drop could be one of the mechanisms behind RYGB induced reduced food intake. (Figure 3)
Figure 3:
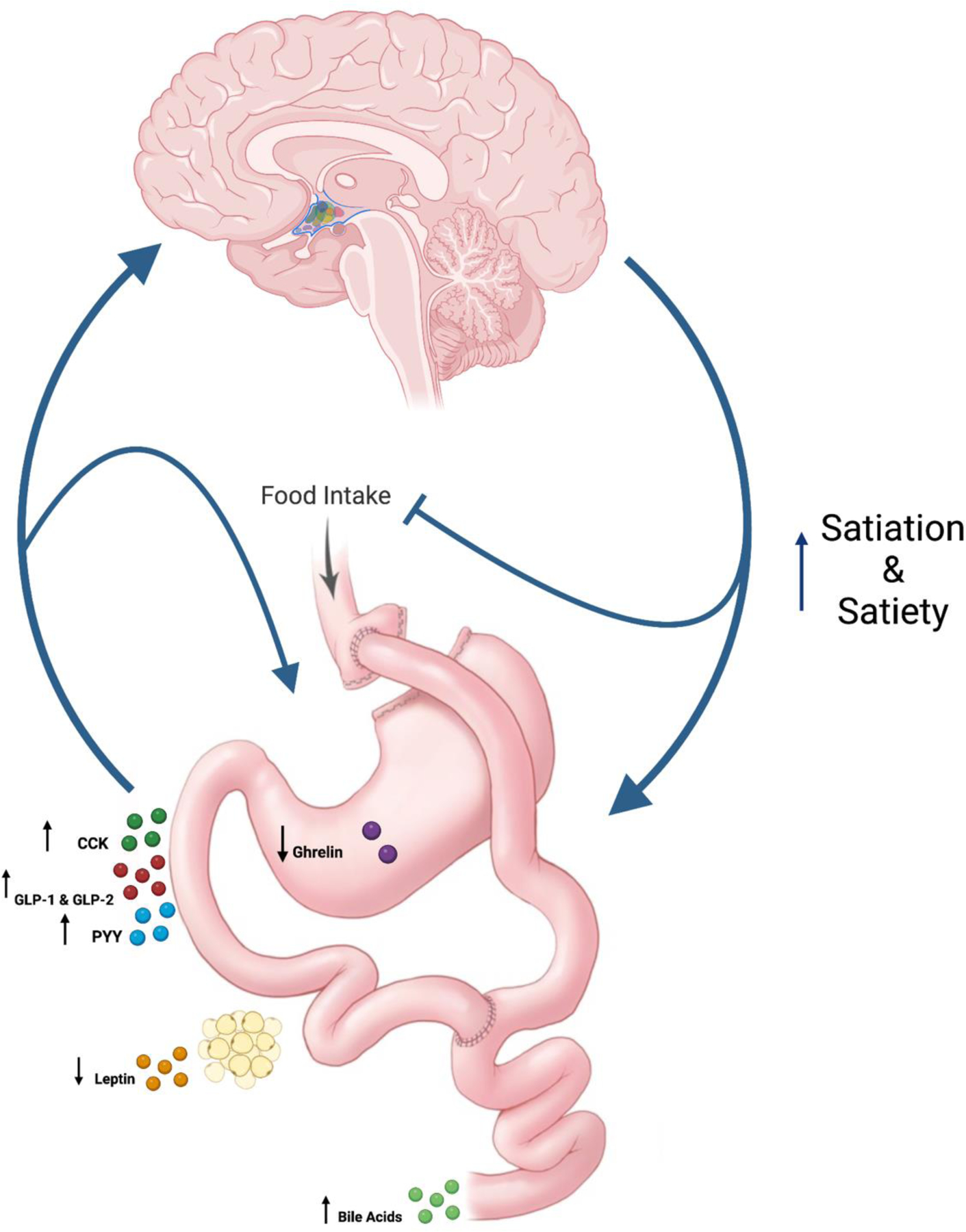
Diagram summary of hormonal changes following RYGB and their interaction with brain-gut-axis. Created with BioRender.com
Expert Opinion
It’s important to note that surgeons still do not have a very good appreciation of why patients are able to lose so much weight following RYGB surgery, when all other techniques for weight loss generally fail in the long term. Physiologic adaptations significantly account for the weight-loss response following RYGB. Changes in EEC density and function from a cellular perspective have not been demonstrated. Almost all the current evidence examining gastric bypass is correlative and descriptive, as there are extremely few mechanistic studies. How cellular adaptations relate to new hormonal postprandial curves could open the field to new therapeutic targets.
EEC have a major impact on gut physiology and food intake regulation. However, our understanding of their physiology in complex states like disease, and post-weight loss intervention status is largely hindered by the relative rarity of EECs, and inaccessibility of methods to isolate and study live cells directly extracted from the gut. Using flow cytometry and single cell technology so isolate EEC from cryopreserved biopsies, and novel methods to determine intracellular signaling pathways, could reveal cellular alterations behind weight loss variability following surgery, as well as new therapeutic targets to treat obesity.
Obesity is one of the multiple diseases associated to leaky gut (171–173). Although one study has shown changes in permeability following RYGB, the generalizability of these findings, and direct impact of said changes is yet to be determined.
Although poorly understood, mucosal mechano- and chemoreceptors interact closely and paracellularly with EEC(13). Studying the mechanism by which suspected EEC changes affect the interaction between EEC and the and the nervous enteric system could reveal new information regarding satiety perception and peripheral gut-neural signaling.
Transecting or preserving vagal branches during the fashioning of the gastric pouch apparently has no impact on weight loss. Due to low gastric pressures following RYGB, it is unlikely to be a determinant factor in signaling the sensation of fullness. On the other hand, rodent models have shown increased sensitivity in celiac branches of the vagus after RYGB which might contribute to reduced meal size. Determining whether vagal signals impact satiety signaling following RYGB in humans, and the mechanism by which it would do so, is an important step to fully characterize adaptations following surgery and develop new hypothesis for potential therapies.
It is important to identify and understand the complex mechanisms by which these homeostatic changes happen after this life-saving procedure. An initial approach towards their identification is recognizing the mechanistic repercussions of bariatric surgery happening in both humans and animal models which likely indicate preserved variables within both species being altered as a possible effect of the operation. Subsequent studies implementing novel techniques to characterize the gaps in the adaptative pathway are required to fully elucidate it in human models.
Highlights:
Postprandial satiety gut hormones increase after RYGB, aiding in weight-loss.
Current studies lack information on enteroendocrine cell adaptations following RYGB.
Most of the evidence in humans is correlative and descriptive not mechanistic.
Animal studies provide valuable insight, but mechanistic human studies are needed.
Acknowledgments:
We thank the support of the Precision Medicine for Obesity Laboratory at Mayo Clinic Rochester Minnesota. Figures were created with Biorender.com. Images were provided by the Mayo Clinic Media Services.
Funding:
Dr. Acosta is supported by NIH (NIH K23-DK114460).
Funding Sources:
The funding sources were not involved in the study design, in the collection, analysis, and interpretation of the study results, in writing the report, or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trial registration: Non-Applicable
Disclosure: Dr. Acosta is a stockholder in Gila Therapeutics, Phenomix Sciences; he served as a consultant for Rhythm Pharmaceuticals, Amgen.
Conflicts of interest:
We have no disclosures, or conflicts of interest.
References:
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief 2020(360):1–8. [PubMed] [Google Scholar]
- 2.Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. New England Journal of Medicine 2017;376(3):254–66. [DOI] [PubMed] [Google Scholar]
- 3.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. New England Journal of Medicine 2019;381(25):2440–50. [DOI] [PubMed] [Google Scholar]
- 4.Waters MG H America’s obesity crisis: the health and economic costs of excess weight Report October, 2018.
- 5.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. Jama 2013;309(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cifuentes L, Acosta A. Homeostatic regulation of food intake. Clin Res Hepatol Gastroenterol 2022;46(2):101794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campos A, Port JD, Acosta A. Integrative Hedonic and Homeostatic Food Intake Regulation by the Central Nervous System: Insights from Neuroimaging. Brain Sciences 2022;12(4):431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piekarski A, Decuypere E, Buyse J, Dridi S. Chenodeoxycholic acid reduces feed intake and modulates the expression of hypothalamic neuropeptides and hepatic lipogenic genes in broiler chickens. General and Comparative Endocrinology 2016;229:74–83. [DOI] [PubMed] [Google Scholar]
- 9.Xie C, Huang W, Young RL, Jones KL, Horowitz M, Rayner CK, et al. Role of Bile Acids in the Regulation of Food Intake, and Their Dysregulation in Metabolic Disease. Nutrients 2021;13(4):1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X, Li JY, Lee A, Lu YX, Zhou SY, Owyang C. Satiety induced by bile acids is mediated via vagal afferent pathways. JCI Insight 2020;5(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinert RE, Feinle-Bisset C, Asarian L, Horowitz M, Beglinger C, Geary N. Ghrelin, CCK, GLP-1, and PYY (3–36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiological reviews 2017;97(1):411–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17(9):1671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinclair P, Brennan DJ, le Roux CW. Gut adaptation after metabolic surgery and its influences on the brain, liver and cancer. Nature reviews Gastroenterology & hepatology 2018;15(10):606–24. [DOI] [PubMed] [Google Scholar]
- 14.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, et al. Gut Hormones as Mediators of Appetite and Weight Loss After Roux-en-Y Gastric Bypass. Annals of Surgery 2007;246(5):780–5. [DOI] [PubMed] [Google Scholar]
- 15.Abellan I, Luján J, Frutos MD, Abrisqueta J, Hernández Q, López V, et al. The influence of the percentage of the common limb in weight loss and nutritional alterations after laparoscopic gastric bypass. Surgery for Obesity and Related Diseases 2014;10(5):829–33. [DOI] [PubMed] [Google Scholar]
- 16.Zorrilla-Nunez LF, Campbell A, Giambartolomei G, Lo Menzo E, Szomstein S, Rosenthal RJ. The importance of the biliopancreatic limb length in gastric bypass: A systematic review. Surgery for Obesity and Related Diseases 2019;15(1):43–9. [DOI] [PubMed] [Google Scholar]
- 17.Wang A, Poliakin L, Sundaresan N, Vijayanagar V, Abdurakhmanov A, Thompson KJ, et al. The role of total alimentary limb length in Roux-en-Y gastric bypass: a systematic review. Surgery for Obesity and Related Diseases 2022;18(4):555–63. [DOI] [PubMed] [Google Scholar]
- 18.Ricardo-Silgado ML, McRae A, Acosta A. Role of Enteroendocrine Hormones in Appetite and Glycemia. Obes Med 2021;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro-Parenti L, Jarry A-C, Cavin J-B, Willemetz A, Le Beyec J, Sannier A, et al. Bariatric surgery induces a new gastric mucosa phenotype with increased functional glucagon-like peptide-1 expressing cells. Nature Communications 2021;12(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorsal T, Wewer Albrechtsen NJ, Christensen MM, Mortensen B, Wandall E, Langholz E, et al. Investigating Intestinal Glucagon After Roux-en-Y Gastric Bypass Surgery. The Journal of Clinical Endocrinology & Metabolism 2019;104(12):6403–16. [DOI] [PubMed] [Google Scholar]
- 21.Spak E, Björklund P, Helander HF, Vieth M, Olbers T, Casselbrant A, et al. Changes in the mucosa of the Roux-limb after gastric bypass surgery. Histopathology 2010;57(5):680–8. [DOI] [PubMed] [Google Scholar]
- 22.Casselbrant A, Elias E, Fändriks L, Wallenius V. Expression of tight-junction proteins in human proximal small intestinal mucosa before and after Roux-en-Y gastric bypass surgery. Surgery for Obesity and Related Diseases 2015;11(1):45–53. [DOI] [PubMed] [Google Scholar]
- 23.Hansen CF, Bueter M, Theis N, Lutz T, Paulsen S, Dalbøge LS, et al. Hypertrophy Dependent Doubling of L-Cells in Roux-en-Y Gastric Bypass Operated Rats. PLOS ONE 2013;8(6):e65696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.le Roux CW, Borg C, Wallis K, Vincent RP, Bueter M, Goodlad R, et al. Gut Hypertrophy After Gastric Bypass Is Associated With Increased Glucagon-Like Peptide 2 and Intestinal Crypt Cell Proliferation. Annals of Surgery 2010;252(1):50–6. [DOI] [PubMed] [Google Scholar]
- 25.Spreckley E, Murphy KG. The L-Cell in Nutritional Sensing and the Regulation of Appetite. Front Nutr 2015;2:23-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg 2012;22(5):740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones B, Sands C, Alexiadou K, Minnion J, Tharakan G, Behary P, et al. The Metabolomic Effects of Tripeptide Gut Hormone Infusion Compared to Roux-en-Y Gastric Bypass and Caloric Restriction. The Journal of Clinical Endocrinology & Metabolism 2021;107(2):e767–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horowitz M, Cook DJ, Collins PJ, Harding PE, Hooper MJ, Walsh JF, et al. Measurement of gastric emptying after gastric bypass surgery using radionuclides. British Journal of Surgery 2005;69(11):655–7. [DOI] [PubMed] [Google Scholar]
- 29.Horowitz M, Collins PJ, Harding PE, Shearman DJ. Gastric emptying after gastric bypass. Int J Obes 1986;10(2):117–21. [PubMed] [Google Scholar]
- 30.Cifuentes L, Camilleri M, Acosta A. Gastric Sensory and Motor Functions and Energy Intake in Health and Obesity-Therapeutic Implications. Nutrients 2021;13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dirksen C, Damgaard M, Bojsen-Møller KN, Jørgensen NB, Kielgast U, Jacobsen SH, et al. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterology & Motility 2013;25(4):346–e255. [DOI] [PubMed] [Google Scholar]
- 32.Uittenbogaart M, Leclercq WKG, Smeele P, van der Linden AN, Luijten AAPM, van Dielen FMH. Reliability and usefulness of upper gastro intestinal contrast studies to assess pouch size in patients with weight loss failure after Roux-en-Y gastric bypass. Acta Chirurgica Belgica 2020;120(5):329–33. [DOI] [PubMed] [Google Scholar]
- 33.Abu Dayyeh BK, Lautz DB, Thompson CC. Gastrojejunal stoma diameter predicts weight regain after Roux-en-Y gastric bypass. Clin Gastroenterol Hepatol 2011;9(3):228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madan AK, Tichansky DS, Phillips JC. Does Pouch Size Matter? Obesity Surgery 2007;17(3):317–20. [DOI] [PubMed] [Google Scholar]
- 35.Athanasiadis DI, Martin A, Kapsampelis P, Monfared S, Stefanidis D. Factors associated with weight regain post-bariatric surgery: a systematic review. Surgical Endoscopy 2021;35(8):4069–84. [DOI] [PubMed] [Google Scholar]
- 36.Rask E, Olsson T, Söderberg S, Johnson O, Seckl J, Holst JJ, et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care 2001;24(9):1640–5. [DOI] [PubMed] [Google Scholar]
- 37.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol 1993;138(1):159–66. [DOI] [PubMed] [Google Scholar]
- 38.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 1999;140(4):1687–94. [DOI] [PubMed] [Google Scholar]
- 39.Roberge JN, Brubaker PL. Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology 1993;133(1):233–40. [DOI] [PubMed] [Google Scholar]
- 40.Holst JJ. The physiology of glucagon-like peptide 1. Physiological reviews 2007;87(4):1409–39. [DOI] [PubMed] [Google Scholar]
- 41.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. The Journal of clinical investigation 1998;101(3):515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Näslund E, Barkeling B, King N, Gutniak M, Blundell JE, Holst JJ, et al. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 1999;23(3):304–11. [DOI] [PubMed] [Google Scholar]
- 43.Verdich C, Flint A, Gutzwiller JP, Näslund E, Beglinger C, Hellström PM, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. The Journal of clinical endocrinology and metabolism 2001;86(9):4382–9. [DOI] [PubMed] [Google Scholar]
- 44.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet (London, England) 2002;359(9309):824–30. [DOI] [PubMed] [Google Scholar]
- 45.Jirapinyo P, Jin DX, Qazi T, Mishra N, Thompson CC. A Meta-Analysis of GLP-1 After Roux-En-Y Gastric Bypass: Impact of Surgical Technique and Measurement Strategy. Obes Surg 2018;28(3):615–26. [DOI] [PubMed] [Google Scholar]
- 46.Yan W, Polidori D, Yieh L, Di J, Wu X, Moreno V, et al. Effects of meal size on the release of GLP-1 and PYY after Roux-en-Y gastric bypass surgery in obese subjects with or without type 2 diabetes. Obes Surg 2014;24(11):1969–74. [DOI] [PubMed] [Google Scholar]
- 47.Harvey EJ, Arroyo K, Korner J, Inabnet WB. Hormone changes affecting energy homeostasis after metabolic surgery. Mt Sinai J Med 2010;77(5):446–65. [DOI] [PubMed] [Google Scholar]
- 48.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg 2006;93(2):210–5. [DOI] [PubMed] [Google Scholar]
- 49.Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. The Journal of clinical endocrinology and metabolism 2005;90(1):359–65. [DOI] [PubMed] [Google Scholar]
- 50.Whitson BA, Leslie DB, Kellogg TA, Maddaus MA, Buchwald H, Billington CJ, et al. Entero-endocrine changes after gastric bypass in diabetic and nondiabetic patients: a preliminary study. J Surg Res 2007;141(1):31–9. [DOI] [PubMed] [Google Scholar]
- 51.Rodieux F, Giusti V, D’Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16(2):298–305. [DOI] [PubMed] [Google Scholar]
- 52.Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. The Journal of clinical endocrinology and metabolism 2008;93(7):2479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007;30(7):1709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morínigo R, Moizé V, Musri M, Lacy AM, Navarro S, Marín JL, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. The Journal of clinical endocrinology and metabolism 2006;91(5):1735–40. [DOI] [PubMed] [Google Scholar]
- 55.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 2007;3(6):597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vidal J, Nicolau J, Romero F, Casamitjana R, Momblan D, Conget I, et al. Long-term effects of Roux-en-Y gastric bypass surgery on plasma glucagon-like peptide-1 and islet function in morbidly obese subjects. The Journal of clinical endocrinology and metabolism 2009;94(3):884–91. [DOI] [PubMed] [Google Scholar]
- 57.Pournaras DJ, Aasheim ET, Bueter M, Ahmed AR, Welbourn R, Olbers T, et al. Effect of bypassing the proximal gut on gut hormones involved with glycemic control and weight loss. Surg Obes Relat Dis 2012;8(4):371–4. [DOI] [PubMed] [Google Scholar]
- 58.Shimizu H, Eldar S, Heneghan HM, Schauer PR, Kirwan JP, Brethauer SA. The effect of selective gut stimulation on glucose metabolism after gastric bypass in the Zucker diabetic fatty rat model. Surgery for Obesity and Related Diseases 2014;10(1):29–35. [DOI] [PubMed] [Google Scholar]
- 59.Eldar S, Heneghan HM, Dan O, Kirwan JP, Schauer PR, Brethauer SA. Gastrostomy tube placement in gastric remnant at gastric bypass: a rat model for selective gut stimulation. Surgery for Obesity and Related Diseases 2013;9(3):442–6. [DOI] [PubMed] [Google Scholar]
- 60.Hansen EN, Tamboli RA, Isbell JM, Saliba J, Dunn JP, Marks-Shulman PA, et al. Role of the foregut in the early improvement in glucose tolerance and insulin sensitivity following Roux-en-Y gastric bypass surgery. American Journal of Physiology-Gastrointestinal and Liver Physiology 2011;300(5):G795–G802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hutch CR, Sandoval D. The Role of GLP-1 in the Metabolic Success of Bariatric Surgery. Endocrinology 2017;158(12):4139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Habegger KM, Heppner KM, Amburgy SE, Ottaway N, Holland J, Raver C, et al. GLP-1R Responsiveness Predicts Individual Gastric Bypass Efficacy on Glucose Tolerance in Rats. Diabetes 2014;63(2):505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.ten Kulve JS, Veltman DJ, Gerdes VEA, van Bloemendaal L, Barkhof F, Deacon CF, et al. Elevated Postoperative Endogenous GLP-1 Levels Mediate Effects of Roux-en-Y Gastric Bypass on Neural Responsivity to Food Cues. Diabetes Care 2017;40(11):1522–9. [DOI] [PubMed] [Google Scholar]
- 64.Drucker DJ. Glucagon-like peptide 2. The Journal of clinical endocrinology and metabolism 2001;86(4):1759–64. [DOI] [PubMed] [Google Scholar]
- 65.Romero F, Nicolau J, Flores L, Casamitjana R, Ibarzabal A, Lacy A, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc 2012;26(8):2231–9. [DOI] [PubMed] [Google Scholar]
- 66.Jacobsen SH, Olesen SC, Dirksen C, Jørgensen NB, Bojsen-Møller KN, Kielgast U, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg 2012;22(7):1084–96. [DOI] [PubMed] [Google Scholar]
- 67.Karra E, Chandarana K, Batterham RL. The role of peptide YY in appetite regulation and obesity. J Physiol 2009;587(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 2002;418(6898):650–4. [DOI] [PubMed] [Google Scholar]
- 69.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. The Journal of clinical investigation 2007;117(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abbott CR, Small CJ, Kennedy AR, Neary NM, Sajedi A, Ghatei MA, et al. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3–36) on food intake. Brain Res 2005;1043(1–2):139–44. [DOI] [PubMed] [Google Scholar]
- 71.Halatchev IG, Cone RD. Peripheral administration of PYY(3–36) produces conditioned taste aversion in mice. Cell Metab 2005;1(3):159–68. [DOI] [PubMed] [Google Scholar]
- 72.Reidelberger R, Haver A, Anders K, Apenteng B. Role of capsaicin-sensitive peripheral sensory neurons in anorexic responses to intravenous infusions of cholecystokinin, peptide YY-(3–36), and glucagon-like peptide-1 in rats. Am J Physiol Endocrinol Metab 2014;307(8):E619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Acosta A, Hurtado MD, Gorbatyuk O, La Sala M, Duncan D, Aslanidi G, et al. Salivary PYY: A Putative Bypass to Satiety. PLOS ONE 2011;6(10):e26137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 2006;147(1):3–8. [DOI] [PubMed] [Google Scholar]
- 75.Ashrafian H, le Roux CW. Metabolic surgery and gut hormones–a review of bariatric entero-humoral modulation. Physiology & behavior 2009;97(5):620–31. [DOI] [PubMed] [Google Scholar]
- 76.Dirksen C, Damgaard M, Bojsen-Møller KN, Jørgensen NB, Kielgast U, Jacobsen SH, et al. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol Motil 2013;25(4):346–e255. [DOI] [PubMed] [Google Scholar]
- 77.Morínigo R, Vidal J, Lacy AM, Delgado S, Casamitjana R, Gomis R. Circulating Peptide YY, Weight Loss, and Glucose Homeostasis After Gastric Bypass Surgery in Morbidly Obese Subjects. Annals of Surgery 2008;247(2). [DOI] [PubMed] [Google Scholar]
- 78.Pocai A Action and therapeutic potential of oxyntomodulin. Mol Metab 2014;3(3):241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moffett RC, Docherty NG, le Roux CW. The altered enteroendocrine reportoire following roux-en-Y-gastric bypass as an effector of weight loss and improved glycaemic control. Appetite 2021;156:104807. [DOI] [PubMed] [Google Scholar]
- 80.Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, et al. Oxyntomodulin Suppresses Appetite and Reduces Food Intake in Humans. The Journal of Clinical Endocrinology & Metabolism 2003;88(10):4696–701. [DOI] [PubMed] [Google Scholar]
- 81.Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, et al. Subcutaneous Oxyntomodulin Reduces Body Weight in Overweight and Obese Subjects: A Double-Blind, Randomized, Controlled Trial. Diabetes 2005;54(8):2390–5. [DOI] [PubMed] [Google Scholar]
- 82.Laferrère B, Swerdlow N, Bawa B, Arias S, Bose M, Oliván B, et al. Rise of Oxyntomodulin in Response to Oral Glucose after Gastric Bypass Surgery in Patients with Type 2 Diabetes. The Journal of Clinical Endocrinology & Metabolism 2010;95(8):4072–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilms B, Ernst B, Thurnheer M, Schmid SM, Spengler CM, Schultes B. Resting energy expenditure after Roux-en Y gastric bypass surgery. Surgery for Obesity and Related Diseases 2018;14(2):191–9. [DOI] [PubMed] [Google Scholar]
- 84.Nielsen MS, Ritz C, Wewer Albrechtsen NJ, Holst JJ, le Roux CW, Sjödin A. Oxyntomodulin and Glicentin May Predict the Effect of Bariatric Surgery on Food Preferences and Weight Loss. The Journal of Clinical Endocrinology & Metabolism 2020;105(4):e1064–e74. [DOI] [PubMed] [Google Scholar]
- 85.Calderon G, McRae A, Rievaj J, Davis J, Zandvakili I, Linker-Nord S, et al. Ileo-colonic delivery of conjugated bile acids improves glucose homeostasis via colonic GLP-1-producing enteroendocrine cells in human obesity and diabetes. EBioMedicine 2020;55:102759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science 1999;284(5418):1365–8. [DOI] [PubMed] [Google Scholar]
- 87.Simmonds WJ, Hofmann AF, Theodor E. Absorption of cholesterol from a micellar solution: intestinal perfusion studies in man. The Journal of clinical investigation 1967;46(5):874–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Camilleri M Peripheral mechanisms in appetite regulation. Gastroenterology 2015;148(6):1219–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mekhjian HS, Phillips SF, Hofmann AF. Colonic absorption of unconjugated bile acids. Digestive Diseases and Sciences 1979;24(7):545–50. [DOI] [PubMed] [Google Scholar]
- 90.Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev 2003;17(13):1581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Camilleri M, Gores GJ. Therapeutic targeting of bile acids. Am J Physiol Gastrointest Liver Physiol 2015;309(4):G209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jansen PL, van Werven J, Aarts E, Berends F, Janssen I, Stoker J, et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis 2011;29(1):48–51. [DOI] [PubMed] [Google Scholar]
- 93.Dutia R, Embrey M, O’Brien S, Haeusler RA, Agénor KK, Homel P, et al. Temporal changes in bile acid levels and 12α-hydroxylation after Roux-en-Y gastric bypass surgery in type 2 diabetes. International Journal of Obesity 2015;39(5):806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gerhard GS, Styer AM, Wood GC, Roesch SL, Petrick AT, Gabrielsen J, et al. A Role for Fibroblast Growth Factor 19 and Bile Acids in Diabetes Remission After Roux-en-Y Gastric Bypass. Diabetes Care 2013;36(7):1859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu T, Bound MJ, Standfield SD, Jones KL, Horowitz M, Rayner CK. Effects of taurocholic acid on glycemic, glucagon-like peptide-1, and insulin responses to small intestinal glucose infusion in healthy humans. The Journal of clinical endocrinology and metabolism 2013;98(4):E718–22. [DOI] [PubMed] [Google Scholar]
- 96.Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, et al. Tauroursodeoxycholic Acid May Improve Liver and Muscle but Not Adipose Tissue Insulin Sensitivity in Obese Men and Women. Diabetes 2010;59(8):1899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. International journal of obesity (2005) 2013;37(12):1553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 2012;153(8):3613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Risstad H, Kristinsson JA, Fagerland MW, le Roux CW, Birkeland KI, Gulseth HL, et al. Bile acid profiles over 5 years after gastric bypass and duodenal switch: results from a randomized clinical trial. Surg Obes Relat Dis 2017;13(9):1544–53. [DOI] [PubMed] [Google Scholar]
- 100.Flynn CR, Albaugh VL, Cai S, Cheung-Flynn J, Williams PE, Brucker RM, et al. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nature Communications 2015;6(1):7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (Lond) 2013;37(12):1553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bhutta HY, Rajpal N, White W, Freudenberg JM, Liu Y, Way J, et al. Effect of Roux-en-Y Gastric Bypass Surgery on Bile Acid Metabolism in Normal and Obese Diabetic Rats. PLOS ONE 2015;10(3):e0122273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keitel V, Görg B, Bidmon HJ, Zemtsova I, Spomer L, Zilles K, et al. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia 2010;58(15):1794–805. [DOI] [PubMed] [Google Scholar]
- 104.Reddy IA, Smith NK, Erreger K, Ghose D, Saunders C, Foster DJ, et al. Bile diversion, a bariatric surgery, and bile acid signaling reduce central cocaine reward. PLOS Biology 2018;16(7):e2006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davis JF, Schurdak JD, Magrisso IJ, Mul JD, Grayson BE, Pfluger PT, et al. Gastric Bypass Surgery Attenuates Ethanol Consumption in Ethanol-Preferring Rats. Biological Psychiatry 2012;72(5):354–60. [DOI] [PubMed] [Google Scholar]
- 106.Grider JR. Role of cholecystokinin in the regulation of gastrointestinal motility. J Nutr 1994;124(8 Suppl):1334s–9s. [DOI] [PubMed] [Google Scholar]
- 107.Ballinger A, McLoughlin L, Medbak S, Clark M. Cholecystokinin is a satiety hormone in humans at physiological post-prandial plasma concentrations. Clin Sci (Lond) 1995;89(4):375–81. [DOI] [PubMed] [Google Scholar]
- 108.Gutzwiller J-P, Degen L, Matzinger D, Prestin S, Beglinger C. Interaction between GLP-1 and CCK-33 in inhibiting food intake and appetite in men. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2004;287(3):R562–R7. [DOI] [PubMed] [Google Scholar]
- 109.Beglinger C, Degen L, Matzinger D, D’Amato M, Drewe J. Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am J Physiol Regul Integr Comp Physiol 2001;280(4):R1149–54. [DOI] [PubMed] [Google Scholar]
- 110.Reidelberger RD, Hernandez J, Fritzsch B, Hulce M. Abdominal vagal mediation of the satiety effects of CCK in rats. Am J Physiol Regul Integr Comp Physiol 2004;286(6):R1005–12. [DOI] [PubMed] [Google Scholar]
- 111.Zhang J, Ritter RC. Circulating GLP-1 and CCK-8 reduce food intake by capsaicin-insensitive, nonvagal mechanisms. Am J Physiol Regul Integr Comp Physiol 2012;302(2):R264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kellum JM, Kuemmerle JF, O’Dorisio TM, Rayford P, Martin D, Engle K, et al. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg 1990;211(6):763–70; discussion 70–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rieu PN, Jansen JB, Hopman WP, Joosten HJ, Lamers CB. Effect of partial gastrectomy with Billroth II or Roux-en-Y anastomosis on postprandial and cholecystokinin-stimulated gallbladder contraction and secretion of cholecystokinin and pancreatic polypeptide. Dig Dis Sci 1990;35(9):1066–72. [DOI] [PubMed] [Google Scholar]
- 114.Pradhan G, Samson SL, Sun Y. Ghrelin: much more than a hunger hormone. Curr Opin Clin Nutr Metab Care 2013;16(6):619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JP. Food fails to suppress ghrelin levels in obese humans. The Journal of clinical endocrinology and metabolism 2002;87(6):2984. [DOI] [PubMed] [Google Scholar]
- 116.Erdmann J, Lippl F, Wagenpfeil S, Schusdziarra V. Differential Association of Basal and Postprandial Plasma Ghrelin With Leptin, Insulin, and Type 2 Diabetes. Diabetes 2005;54(5):1371–8. [DOI] [PubMed] [Google Scholar]
- 117.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 2002;346(21):1623–30. [DOI] [PubMed] [Google Scholar]
- 118.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33(7):786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin E, Gletsu N, Fugate K, McClusky D, Gu LH, Zhu J-L, et al. The Effects of Gastric Surgery on Systemic Ghrelin Levels in the Morbidly Obese. Archives of Surgery 2004;139(7):780–4. [DOI] [PubMed] [Google Scholar]
- 120.Tymitz K, Engel A, McDonough S, Hendy MP, Kerlakian G. Changes in ghrelin levels following bariatric surgery: review of the literature. Obes Surg 2011;21(1):125–30. [DOI] [PubMed] [Google Scholar]
- 121.Xu H-C, Pang Y-C, Chen J-W, Cao J-Y, Sheng Z, Yuan J-H, et al. Systematic Review and Meta-analysis of the Change in Ghrelin Levels After Roux-en-Y Gastric Bypass. Obesity Surgery 2019;29(4):1343–51. [DOI] [PubMed] [Google Scholar]
- 122.Tamboli RA, Breitman I, Marks-Shulman PA, Jabbour K, Melvin W, Williams B, et al. Early weight regain after gastric bypass does not affect insulin sensitivity but is associated with elevated ghrelin. Obesity 2014;22(7):1617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dimaraki EV, Jaffe CA. Role of endogenous ghrelin in growth hormone secretion, appetite regulation and metabolism. Reviews in Endocrine and Metabolic Disorders 2006;7(4):237–49. [DOI] [PubMed] [Google Scholar]
- 124.Groschl M, Uhr M, Kraus T. Evaluation of the comparability of commercial ghrelin assays. Clinical chemistry 2004;50(2):457–8. [DOI] [PubMed] [Google Scholar]
- 125.Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. The Lancet 2021;398(10295):143–55. [DOI] [PubMed] [Google Scholar]
- 126.Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. New England Journal of Medicine 2021;385(6):503–15. [DOI] [PubMed] [Google Scholar]
- 127.Thomas MK, Nikooienejad A, Bray R, Cui X, Wilson J, Duffin K, et al. Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes. The Journal of Clinical Endocrinology & Metabolism 2020;106(2):388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. American Journal of Physiology-Endocrinology And Metabolism 2003;284(4):E671–E8. [DOI] [PubMed] [Google Scholar]
- 129.Woods SC, Lutz TA, Geary N, Langhans W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philosophical Transactions of the Royal Society B: Biological Sciences 2006;361(1471):1219–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jones B, Tan T, Bloom S. Minireview: glucagon in stress and energy homeostasis. Endocrinology 2012;153(3):1049–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Galsgaard KD, Pedersen J, Knop FK, Holst JJ, Wewer Albrechtsen NJ. Glucagon receptor signaling and lipid metabolism. Frontiers in physiology 2019;10:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Finan B, Capozzi ME, Campbell JE. Repositioning glucagon action in the physiology and pharmacology of diabetes. Diabetes 2020;69(4):532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carr RD, Larsen MO, Jelic K, Lindgren O, Vikman J, Holst JJ, et al. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. The Journal of Clinical Endocrinology & Metabolism 2010;95(2):872–8. [DOI] [PubMed] [Google Scholar]
- 134.Rauch T, Graefe-Mody U, Deacon CF, Ring A, Holst JJ, Woerle H-J, et al. Linagliptin increases incretin levels, lowers glucagon, and improves glycemic control in type 2 diabetes mellitus. Diabetes Therapy 2012;3(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Langhans W, Zeiger U, Scharrer E, Geary N. Stimulation of feeding in rats by intraperitoneal injection of antibodies to glucagon. Science 1982;218(4575):894–6. [DOI] [PubMed] [Google Scholar]
- 136.Pocai A, Carrington PE, Adams JR, Wright M, Eiermann G, Zhu L, et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes 2009;58(10):2258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hope DCD, Vincent ML, Tan TMM. Striking the Balance: GLP-1/Glucagon Co-Agonism as a Treatment Strategy for Obesity. Frontiers in Endocrinology 2021;12. [DOI] [PMC free article] [PubMed]
- 138.Yang C, Brecht J, Weiß C, Reissfelder C, Otto M, Buchwald JN, et al. Serum Glucagon, Bile Acids, and FGF-19: Metabolic Behavior Patterns After Roux-en-Y Gastric Bypass and Vertical Sleeve Gastrectomy. Obesity Surgery 2021;31(11):4939–46. [DOI] [PubMed] [Google Scholar]
- 139.Salehi M, Gastaldelli A, D’alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. The Journal of Clinical Endocrinology & Metabolism 2014;99(6):2008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Salehi M, Gastaldelli A, D’Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 2014;146(3):669–80. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Goldfine AB, Mun E, Devine E, Bernier R, Baz-Hecht M, Jones D, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. The Journal of Clinical Endocrinology & Metabolism 2007;92(12):4678–85. [DOI] [PubMed] [Google Scholar]
- 142.Dupre J, Ross S, Watson D, Brown J. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. The Journal of Clinical Endocrinology & Metabolism 1973;37(5):826–8. [DOI] [PubMed] [Google Scholar]
- 143.Holst J, Ørskov C, Vagn Nielsen O, Schwartz T. Truncated glucagon‐like peptide I, an insulin‐releasing hormone from the distal gut. FEBS letters 1987;211(2):169–74. [DOI] [PubMed] [Google Scholar]
- 144.Nauck MA, Heimesaat MM, Orskov C, Holst J, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. The Journal of clinical investigation 1993;91(1):301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Beck B, Max J-P. Gastric inhibitory polypeptide enhancement of the insulin effect on fatty acid incorporation into adipose tissue in the rat. Regulatory peptides 1983;7(1):3–8. [DOI] [PubMed] [Google Scholar]
- 146.Oben J, Morgan L, Fletcher J, Marks V. Effect of the entero-pancreatic hormones, gastric inhibitory polypeptide and glucagon-like polypeptide-1 (7–36) amide, on fatty acid synthesis in explants of rat adipose tissue. Journal of Endocrinology 1991;130(2):267–72. [DOI] [PubMed] [Google Scholar]
- 147.Hauner H, Glatting G, Kaminska D, Pfeiffer E-F. Effects of gastric inhibitory polypeptide on glucose and lipid metabolism of isolated rat adipocytes. Annals of nutrition and metabolism 1988;32(5–6):282–8. [DOI] [PubMed] [Google Scholar]
- 148.Trümper A, Trümper K, Trusheim H, Arnold R, Göke B, Hörsch D. Glucose-dependent insulinotropic polypeptide is a growth factor for β (INS-1) cells by pleiotropic signaling. Molecular endocrinology 2001;15(9):1559–70. [DOI] [PubMed] [Google Scholar]
- 149.Zhou J, Wang X, Pineyro MA, Egan JM. Glucagon-like peptide 1 and exendin-4 convert pancreatic AR42J cells into glucagon-and insulin-producing cells. Diabetes 1999;48(12):2358–66. [DOI] [PubMed] [Google Scholar]
- 150.Adriaenssens AE, Biggs EK, Darwish T, Tadross J, Sukthankar T, Girish M, et al. Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell metabolism 2019;30(5):987–96. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.NamKoong C, Kim MS, Jang B-T, Lee YH, Cho Y-M, Choi HJ. Central administration of GLP-1 and GIP decreases feeding in mice. Biochemical and biophysical research communications 2017;490(2):247–52. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Q, Delessa CT, Augustin R, Bakhti M, Colldén G, Drucker DJ, et al. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell metabolism 2021;33(4):833–44. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gao Z, Yang J, Liang Y, Yang S, Zhang T, Gong Z, et al. Changes in Gastric Inhibitory Polypeptide (GIP) After Roux-en-Y Gastric Bypass in Obese Patients: a Meta-analysis. Obes Surg 2022. [DOI] [PubMed]
- 154.Qi D, Cai K, Wang O, Li Z, Chen J, Deng B, et al. Fatty acids induce amylin expression and secretion by pancreatic β-cells. American Journal of Physiology-Endocrinology and Metabolism 2010;298(1):E99–E107. [DOI] [PubMed] [Google Scholar]
- 155.Ogawa A, Harris V, McCorkle SK, Unger RH, Luskey KL. Amylin secretion from the rat pancreas and its selective loss after streptozotocin treatment. The Journal of clinical investigation 1990;85(3):973–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kanatsuka A, Makino H, Ohsawa H, Tokuyama Y, Yamaguchi T, Yoshida S, et al. Secretion of islet amyloid polypeptide in response to glucose. FEBS letters 1989;259(1):199–201. [DOI] [PubMed] [Google Scholar]
- 157.Asmar M, Bache M, Knop FK, Madsbad S, Holst JJ. Do the actions of glucagon-like peptide-1 on gastric emptying, appetite, and food intake involve release of amylin in humans? The Journal of Clinical Endocrinology & Metabolism 2010;95(5):2367–75. [DOI] [PubMed] [Google Scholar]
- 158.Lutz TA. The role of amylin in the control of energy homeostasis. American journal of physiology-regulatory, integrative and comparative physiology 2010;298(6):R1475–R84. [DOI] [PubMed] [Google Scholar]
- 159.Geary N A new way of looking at eating. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2005;288(6):R1444–R6. [DOI] [PubMed] [Google Scholar]
- 160.Lutz TA, Geary N. Gastrointestinal factors in appetite and food intake-animal research. Appetite and Food Intake, edited by Harris RBS and Mattes RD Boca Raton, FL: CRC. 2008:163–86. [Google Scholar]
- 161.Potes CS, Lutz TA. Brainstem mechanisms of amylin-induced anorexia. Physiology & behavior 2010;100(5):511–8. [DOI] [PubMed] [Google Scholar]
- 162.Beglinger S, Meyer‐Gerspach AC, Graf S, Zumsteg U, Drewe J, Beglinger C, et al. Effect of a test meal on meal responses of satiation hormones and their association to insulin resistance in obese adolescents. Obesity 2014;22(9):2047–52. [DOI] [PubMed] [Google Scholar]
- 163.Kautzky-Willer A, Thomaseth K, Pacini G, Clodi M, Ludvik B, Streli C, et al. Role of islet amyloid polypeptide secretion in insulin-resistant humans. Diabetologia 1994;37(2):188–94. [DOI] [PubMed] [Google Scholar]
- 164.Blackard WG, Clore JN, Kellum JM. Amylin/insulin secretory ratios in morbidly obese man: inverse relationship with glucose disappearance rate. The Journal of Clinical Endocrinology & Metabolism 1994;78(5):1257–60. [DOI] [PubMed] [Google Scholar]
- 165.Hanabusa T, Kubo K, Oki C, Nakano Y, Okai K, Sanke T, et al. Islet amyloid polypeptide (IAPP) secretion from islet cells and its plasma concentration in patients with non-insulin-dependent diabetes mellitus. Diabetes research and clinical practice 1992;15(1):89–96. [DOI] [PubMed] [Google Scholar]
- 166.Jacobsen S, Olesen S, Dirksen C, Jørgensen N, Bojsen-Møller K, Kielgast U, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obesity surgery 2012;22(7):1084–96. [DOI] [PubMed] [Google Scholar]
- 167.Nussbaumer R, Meyer-Gerspach AC, Peterli R, Peters T, Beglinger C, Chiappetta S, et al. First-Phase Insulin and Amylin after Bariatric Surgery: A Prospective Randomized Trial on Patients with Insulin Resistance or Diabetes after Gastric Bypass or Sleeve Gastrectomy. Obesity Facts 2020;13(6):584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang J-W, Chen P-Y, Huang H-H, Yeh C, Chen S-C, Lee W-J, et al. Change of plasma amylin after bariatric surgery challenged by oral glucose is associated with remission of type 2 diabetes mellitus. Journal of the Chinese Medical Association 2021;84(11). [DOI] [PubMed] [Google Scholar]
- 169.Nannipieri M, Baldi S, Mari A, Colligiani D, Guarino D, Camastra S, et al. Roux-en-Y Gastric Bypass and Sleeve Gastrectomy: Mechanisms of Diabetes Remission and Role of Gut Hormones. The Journal of Clinical Endocrinology & Metabolism 2013;98(11):4391–9. [DOI] [PubMed] [Google Scholar]
- 170.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 2010;151(4):1588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Camilleri M Leaky gut: mechanisms, measurement and clinical implications in humans. Gut 2019;68(8):1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Camilleri M, Vella A. What to do about the leaky gut. Gut 2022;71(2):424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Frazier TH, DiBaise JK, McClain CJ. Gut Microbiota, Intestinal Permeability, Obesity-Induced Inflammation, and Liver Injury. Journal of Parenteral and Enteral Nutrition 2011;35(5S):14S–20S. [DOI] [PubMed] [Google Scholar]


