Figure 1. How cancer cells make and respond to IFN-I.
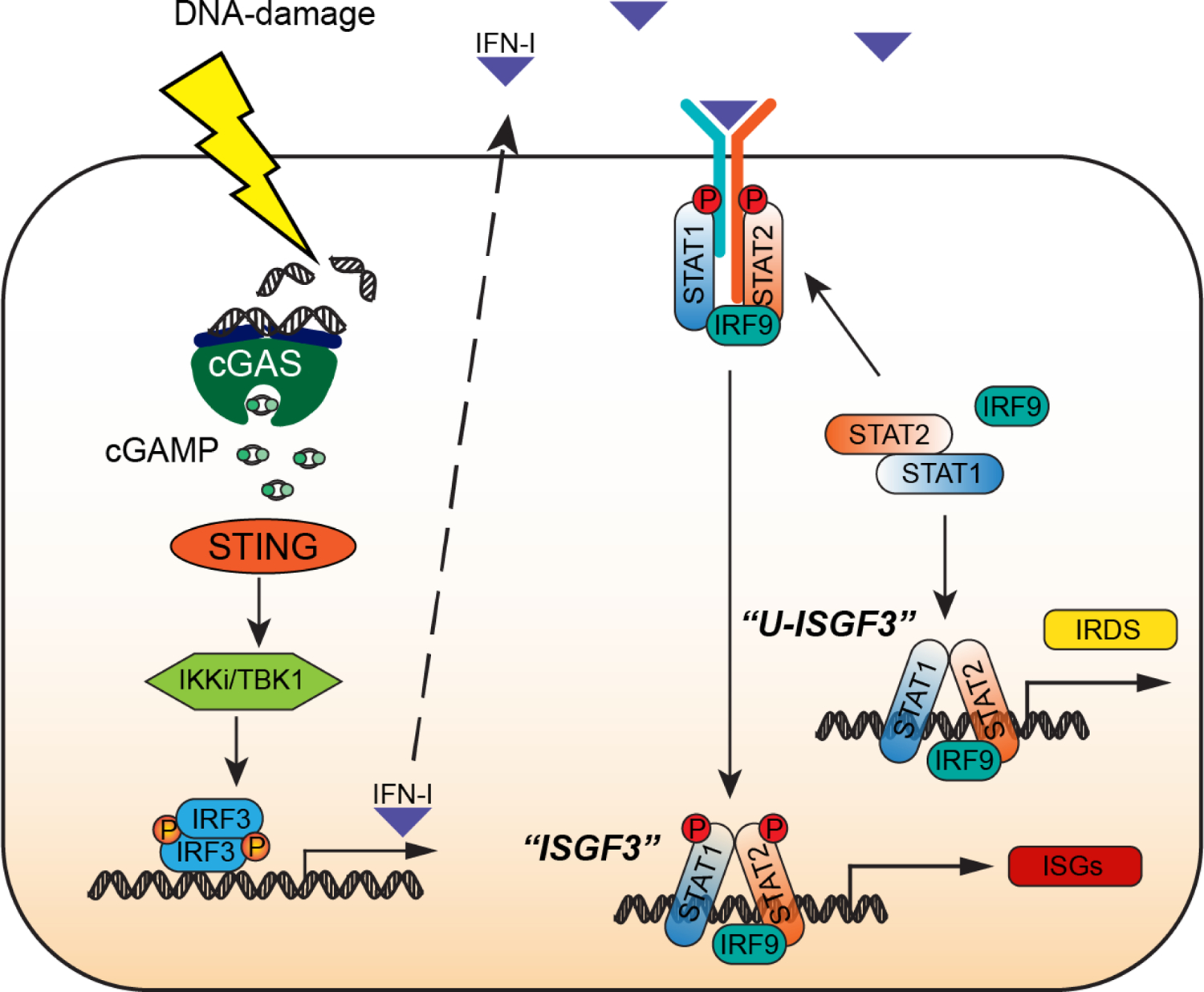
Activation of the cGAS-STING pathway by DNA-damaging chemotherapeutic agents (shown) or in response to endogenous cytoplasmic DNA (not shown) leads to robust production of IFN-I. Newly synthesized autocrine IFN-I drives the expression of interferon-stimulated genes (ISGs) by using the receptor-bound kinases, Janus Kinase 1 (JAK1) and Tyrosine Kinase 2 (TYK2), to phosphorylate and thus activate STAT1 and STAT2 on specific tyrosine residues (Y701 for STAT1, Y690 for STAT2), followed by the formation of tyrosine phosphorylated STAT1:STAT2 heterodimers that associate with IRF9 to form ISGF3, the major transcription factor driving the response to IFN-I. STAT1, STAT2, and IRF9 are themselves ISGs, so that these three proteins are present at high levels in cells that are exposed to IFN-I constitutively, forming an ISGF3 complex that lacks tyrosine phosphorylation (U-ISGF3) which, in turn, drives the expression of a subset of ISGs, the Interferon-Related DNA Damage Resistance Signature (IRDS). Since the conformation of U-ISGF3 is not yet known, the representation in the figure should be regarded as schematic only.
