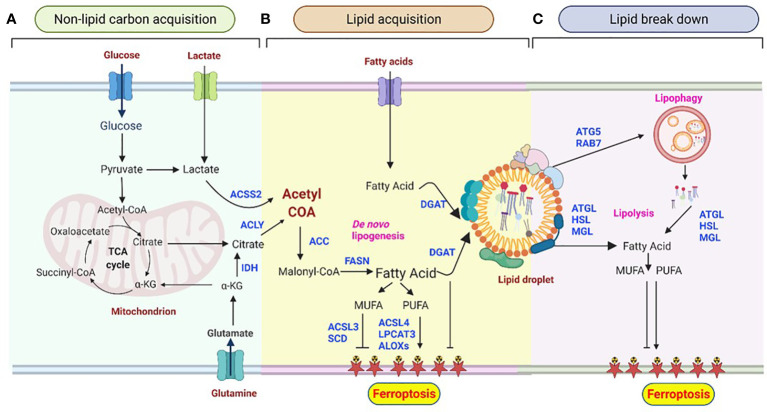
Figure 1.
Lipid droplet metabolism is central to shaping the ferroptotic response. (A) Non-lipid carbon acquisition of carbon in cancer cells. Glucose- and glutamine-derived citrate, which results from increased glycolysis and glutaminolysis, is first converted to acetyl-coenzyme A (acetyl-CoA) by ATP-citrate lyase (ACLY). Acetyl-CoA can also be derived from acetate. (B) De novo lipogenesis (DNL) and esterification in ferroptosis. Acetyl-CoA is converted to malonyl-CoA by acetyl-CoA carboxylase (ACC) and condensed by fatty acid synthase (FAS). Acyl-CoA synthetase long-chain 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) mediate the production of polyunsaturated fatty acids (PUFAs), which are essential for the induction of ferroptosis. In contrast, acyl-CoA synthetase long-chain 3 (ACSL3) and stearoyl CoA desaturase (SCD) contribute to the synthesis of monounsaturated fatty acids (MUFAs), leading to ferroptosis resistance. Arachidonate lipoxygenases (ALOXs) catalyze the stereospecific insertion of oxygen into PUFAs, thereby promoting ferroptosis. Diacylglycerol acyltransferase (DGAT1/2)-mediated triglyceride synthesis and lipid droplet formation act as a sink for free fatty acids, thus preventing their peroxidation.(C) Lipid degradation in ferroptosis. The selective degradation of lipid droplets by member RAS oncogene family 7 (RAB7A)- and adipocyte triglyceride lipase (ATGL)-related lipophagy increases the production of free fatty acids for subsequent ferroptosis. Lipolysis may provide PUFAs, thus stimulating lipid peroxidation and sensitizing cells to ferroptosis. Lipases may also provide MUFAs that reduce the abundance of oxidizable PUFAs in membranes, thereby restricting lipid peroxidation and ferroptosis. Lipid droplets act as buffers of lipid flux and release, thereby emerging as master regulators of ferroptotic sensitivity.