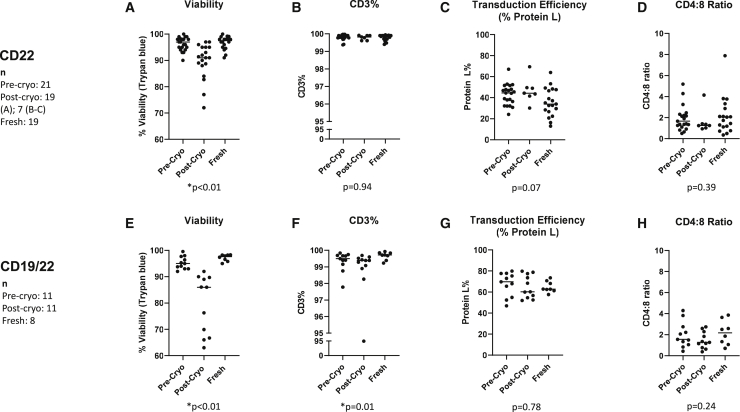
Figure 2.
Manufacturing characteristics of CAR T cells before and after cryopreservation
Viability, CD3 percent, transduction efficiency (based on Protein L percent), and CD4:8 ratios are shown for pre-cryopreservation, post-cryopreservation, and fresh samples. Viability was tested for all samples prior to infusion. Post-thaw CD3 percent, Protein L percent, and CD4:8 ratios were only tested in a subset of anti-CD22 CAR T cells (n = 7) and for all cryopreserved anti-CD19/22 CAR T cells (n = 11). The results of testing anti-CD22-CAR T cell products are shown in (A)–(D), and those of anti-CD19/CD22 bispecific CAR T cell products are shown in (E)–(H). (A) and (E) show the viability, (B) and (F) show CD3 percent, (C) and (G) the percent of cells expressing Protein L, and (D) and (H) the ratio of CD4:CD8-expressing cells. p values represent a comparison between freshly infused and post-cryopreservation products. For pre- and post-cryopreservation comparisons, please refer to Figure S2.