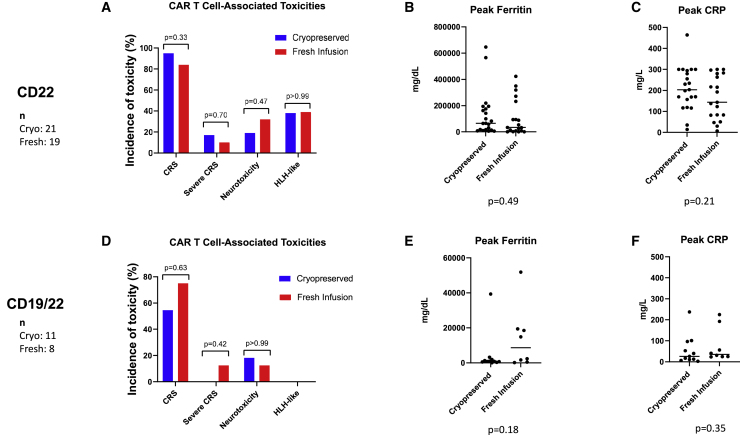
Figure 4.
CAR T cell-associated toxicities for patients receiving cryopreserved versus fresh CAR T cells
CAR T cell-associated toxicities in patients who received anti-CD22 CAR T cells are shown in (A) and for those who received anti-CD19/CD22 CAR T cells in (D). The percentage of patients experiencing any grade of CRS, severe CRS defined as grades 3 or 4, neurotoxicity, or HLH are shown. The brackets show the p values for comparison of patients receiving cryopreserved and fresh products. Following the infusion of CAR T cells, ferritin and C-reactive protein (CRP) were measured for all patients. (B) shows peak ferritin levels and (C) shows peak CRP levels in patients receiving anti-CD22 CAR T cells (n = 40). For patients receiving anti-CD19/CD22 CAR T cells (n = 19), peak ferritin levels are shown in (E) and peak CRP levels in (F). Please note that scales on y axes differ between different cohorts in order to optimize viewing of individual data points.