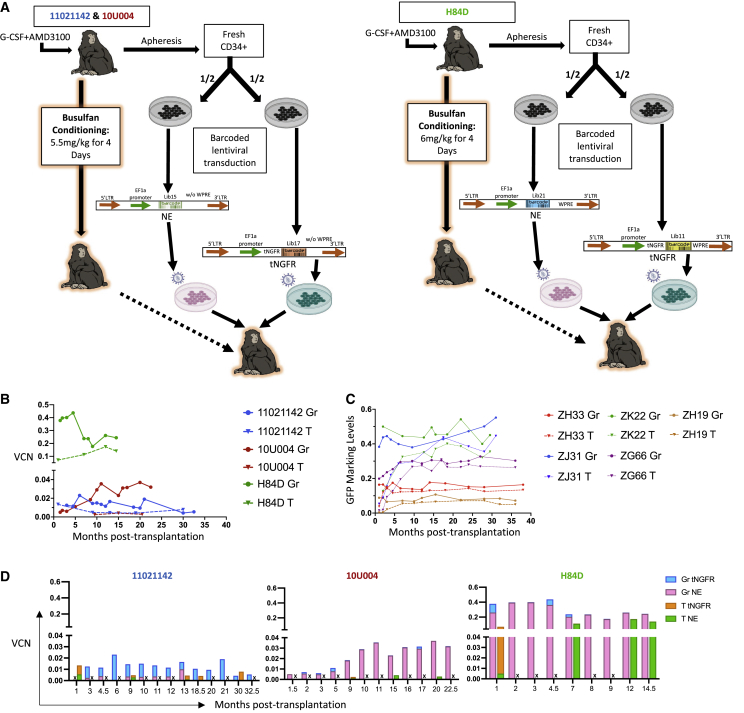
Figure 1.
Experimental design and marking summary
(A) Animal-specific conditioning and transplantation schema. Mobilized peripheral blood (PB) CD34+ cells from each animal were split into two equal aliquots and each fraction was transduced with lentiviral barcode library containing an elongation factor 1-α (EF1-α) promoter, with no expressed transgene marker (NE) or with a truncated nerve growth factor receptor marker gene (tNGFR), and distinguished by a unique library ID. After transduction, both fractions were cryopreserved during busulfan conditioning, then thawed and infused intravenously into the autologous macaque. Animals 11021142 and 10U004 received 5.5 mg/kg of intravenous busulfan for 4 consecutive days, while animal H84D received 6.0 mg/kg for 4 consecutive days. (B) Vector copy number (VCN) of PB granulocytes (Gr) and T cells from the three busulfan animals over time determined by digital droplet PCR (ddPCR) using a probe targeting to the RRE region shared by both vectors, and a probe detecting the housekeeping gene TERT. (C) Summary of GFP marking levels of PB Gr and T lineages from five macaques conditioned with myeloablative TBI and transplanted with CD34+ HSPCs transduced with cop-GFP expressing lentiviral barcode libraries. Solid lines: Gr, dotted lines: T cells, with each color representing an individual animal. (D) Molecular marking level comparisons of PB Gr and T between NE and tNGFR barcode libraries in the three busulfan animals over time. The total VCN determined in 1B (VCN) was multiplied by the fraction of reads retrieved carrying each specific library ID from Illumina sequencing to calculate the VCN attributed to the tNGFR and the NE vectors. “X” in the bar graphs indicates the sample for that time point is unavailable, thus no results are provided.