Abstract
Purpose:
Active surveillance (AS) is increasingly used as a management strategy for localized prostate cancer. Coffee intake has been associated with lower prostate cancer incidence; and we assessed whether coffee was associated with disease progression in men on AS.
Materials and Methods:
Patients with newly diagnosed Gleason score (GS) 6 or 7 prostate cancer were enrolled on a prospective AS protocol for at least 6 months and completed a baseline dietary assessment (n=411). The AS protocol included a biennial monitoring regimen with disease progression defined as an increase in GS. Cox proportional hazards models were used to evaluate associations of coffee intake with progression-free survival. Patient genotype in the caffeine metabolism-related SNP rs762551 was also evaluated.
Results:
Median follow-up was 36 months (range 6 – 126), and 76/411 (18.5%) had GS progression. In the multivariable model adjusting for PSA, age and tumor length, compared to 0 cups/day, <1 cup (HR 0.85, 95%CI 0.40–1.71), 1–1.9 cups (HR 0.64, 95%CI 0.29–1.43), 2–3.9 cups (HR 0.71, 95%CI 0.35–1.47), and ≥4 cups (HR 1.67, 95%CI 0.81–3.45) were not significantly associated with progression-free survival (P for non-linearity = 0.01). Patients with low/moderate coffee intake and the AA “fast caffeine metabolizer” genotype were less likely to experience grade progression, as compared to non-consumers (HR 0.36, 95% CI 0.15–0.88, P=0.03).
Conclusions:
Low to moderate coffee intake appears safe in men on AS for localized prostate cancer. Further work is needed to determine if high consumption is associated with shorter progression-free survival in sensitive groups.
Keywords: Prostatic neoplasms, coffee, caffeine, genetic variation, risk
INTRODUCTION
The vast majority of men diagnosed with prostate cancer are found to have localized disease, and over 50% of these may be eligible for active surveillance (AS) based on conservative criteria.1 The use and adaptation of AS is increasing,2 likely reflecting the low rate of disease progression seen in low-risk tumors3 and known health-related quality of life changes associated with radical prostate cancer treatment.4
While multiple clinical and patient-related factors such as serum markers (ex. PSA), biopsy results (pathologic Gleason score, tumor volume) and age have been associated with disease progression on surveillance5, few other modifiable factors have been identified. Coffee consumption has been linked to lower risk of prostate and other tumors, as well as lower risk of aggressive prostate cancer.6–8 It is hypothesized that coffee may have a protective effect through decreased insulin-like growth factor9 and/or systemic inflammation.10 However, the effect of coffee consumption on prostate cancer progression following diagnosis is unknown.
We assessed diet, including coffee intake, as part of a prospective protocol for men on AS for localized prostate cancer. Based on prior protective evidence in prostate cancer incidence6 and locally advanced colorectal cancer patient outcomes11, we hypothesized that regular coffee intake would be associated with improved progression-free survival in localized prostate cancer patients followed on AS. We also explored the effects of caffeine and other beverage sources, as well as variation in caffeine metabolism genotype.
METHODS
Study design and population
Patients in this prospective clinical cohort included men diagnosed with localized prostate cancer and enrolled on an AS trial protocol between February 2006 and February 2012 (n=560). All patients were asked to complete a baseline dietary assessment (501 complied) and 486 patients provided complete data for the current analysis. Of these, 411 patients remained on active surveillance for at least 6 consecutive months and were included in the analysis.
Surveillance protocol and outcomes assessment
The surveillance protocol was conducted by a multidisciplinary team of urologists, radiation oncologists and medical oncologists, was approved by the Institutional Review Board, and is registered on clinicaltrials.gov (NCT00490763). Protocol criteria, including surveillance frequency and details regarding upstaging were adhered to as previously described.12 In summary, with rare exception, patients underwent confirmatory biopsy at study entry. They were then evaluated biannually with digital rectal exam and laboratory studies (serum PSA, testosterone). All biopsies were performed using an 11-core trans-rectal ultrasound-guided scheme.13 Biopsies were repeated every 1–2 years; if one was negative, then the following year’s was omitted. Patients who had an increase in tumor volume or Gleason increase were recommended to undergo treatment, though patients who wished to remain on AS were allowed to do so if approved by their treating physician. Patients were followed until disease progression, treatment, loss to follow-up, elective removal, death or 12/31/2016 (study censor date), whichever came first. Our outcome of interest was time to grade progression, defined as any increase in Gleason score following confirmatory biopsy. Patient enrollment was not restricted based on medication or supplement use, including 5-alpha reductase inhibitors.
Dietary Assessment
A comprehensive food frequency questionnaire (FFQ) was used to collect patients’ usual food and beverage intake (frequency and portion size) as previously described.14 FFQs were reviewed and coded by trained registered dietitians. Total gram weight per day of regular (caffeinated) coffee and soft drinks; black, green, and herbal tea; and milk, cream, sugar or honey added to coffee/tea were assessed. Intake of total energy, caffeine, and other nutrients were estimated using the US Department of Agriculture Food and Nutrient Database for Dietary Studies (http://www.ars.usda.gov/ba/bhnrc/ndl). Individuals with extreme total energy intake distant from other observations (beyond twice the interquartile range of Box-Cox transformed intake) were excluded from the analysis.
rs762551 Genotyping
Prior evidence indicates that the −163A>C (rs762551) single nucleotide polymorphism (SNP) alters CYP1A2 enzyme activity and can categorize individuals as “fast” or “slow” caffeine metabolizers.15 To further investigate the role that caffeine metabolism may play in modulating the relationship between coffee and prostate cancer progression, we obtained genotype data for this SNP. Genotyping was performed using the OncoArray gene chip (Illumina, San Diego, CA) for a subset of men. Since rs762551 was not directly genotyped, imputation was performed in a two-stage procedure using SHAPEIT2 to derive phased genotypes, and IMPUTE2 to perform imputation of phased data by PRACTICAL16 based on the 1000 genomes release 3 reference panel. The imputed R2 value of this SNP was >0.99. If the estimated dosage was >= 1.9, then the AA genotype was called. If the dosage was <= 0.1, then the CC genotype was called. If the dosage was between 0.9 and 1.10, then the AC genotype was called.
Statistical Analysis
Regular (caffeinated) coffee intake was categorized a priori into groups similar to a previous analysis of colorectal cancer patients11, using 230 daily grams of intake as the equivalent of a single cup (0 cups, <1cup, 1–1.9 cups, 2–3.9 cups, and ≥4 cups per day). Survival curves for progression-free survival (PFS) across coffee category were generated using the Kaplan-Meier method. We evaluated the association of coffee consumption with time to progression using person-years as the underlying time metric in Cox proportional hazards models adjusted for other pertinent factors. We confirmed that the proportional hazards assumption was met through assessment of interaction terms for the exposures with follow-up time. Hazard ratios (HR), 95% confidence intervals (CI) and P values for linear and non-linear trend are reported across strata of coffee intake, with non-consumers representing the referent group. In addition to the base (age-adjusted) model, we assessed models adjusted for clinicopathologic factors (base + clinical) including those associated with progression free survival in this cohort (age, PSA, and summation tumor length from baseline and confirmatory biopsy). We further assessed a model adjusted for key lifestyle and demographic prostate cancer risk factors, including smoking status, race, BMI, alcohol and statin use. Additional adjustment for total energy intake (standardization per 1,000 kcal plus a continuous covariate for energy), multivitamin or other supplement use, history of diabetes, and hypertensive status did not appreciably change the estimated effects observed. To assess the deviation from linear trend, we evaluated a quadratic term for coffee intake (coffee intake-squared) in addition to the continuous variable for coffee intake within each of the models. Missing data for covariates were excluded in relevant analyses, though this occurred <5% of the time. In exploratory analyses, we further evaluated whether observed associations varied by smoking status, BMI, statin use, circulating testosterone level, alcohol drinking status, and rs762551 (caffeine metabolism) genotype. Given that only caffeinated coffee intake was assessed, we also examined associations of total caffeine intake and intake of other caffeinated and decaffeinated beverages with progression. All statistical tests were 2-sided and were considered statistically significant at P<0.05. Analyses were performed using STATA version 13.1 (StataCorp, College Station, TX).
RESULTS
Baseline characteristics by frequency of regular coffee consumption are displayed in Table 1. Median coffee consumption was 1.03 cups/day (range 0–10.65 cups/day); and 79% of patients (326/411) drank some coffee weekly. A subset of patients (68.6%; 282/411) additionally completed a 6-month follow-up FFQ, which demonstrated little to no change in coffee intake (Supplementary Figure 1); however, heavy coffee drinkers were the group most likely to reduce their consumption (data not show).
Table 1:
Selected baseline characteristics of men with localized prostate cancer on active surveillance (n=411)* by coffee intake
| Characteristic | 0 cups/day N=74 |
<1cup/day N=85 |
1–1.9 cups/day N=87 |
2–3.9 cups/day N=106 |
≥4 cups/day N=59 |
|---|---|---|---|---|---|
| Age (mean, STD) | 63.3 (8.7) | 64.9 (9.2) | 64.6 (8.9) | 65.1 (7.8) | 63.5 (7.1) |
| Race | |||||
| White | 56 (75.7) | 66 (77.6) | 73 (83.9) | 93 (87.7) | 53 (89.8) |
| Black | 12 (16.2) | 9 (10.6) | 5 (5.7) | 5 (4.7) | 2 (3.4) |
| Other/unknown | 6 (8.1) | 10 (11.8) | 9 ( 10.3) | 8 (7.5) | 4 (6.8) |
| PSA (mean, STD) | 4.1 (2.3) | 4.4 (2.5) | 4.5 (3.1) | 3.6 (2.2) | 4.3 (2.8) |
| Summation tumor length (mm) | 3.1 (4.4) | 4.1 (5.3) | 2.9 (3.5) | 4.2 (6.8) | 2.9 (2.6) |
| Baseline core positivitiy (N,%) | |||||
| Single | 54 (73.0) | 58 (68.2) | 62 (71.3) | 79 (74.5) | 45 (76.3) |
| Multiple | 20 (27.0) | 27 (31.8) | 25 (28.7) | 27 (25.5) | 14 (23.7) |
| Baseline Gleason Score (N,%) | |||||
| Gleason 6 | 66 (89.2) | 68 (80.0) | 79 (90.8) | 96 (90.6) | 49 (83.1) |
| Gleason 7 | 8 (10.8) | 17 (20.0) | 8 (9.2) | 10 (9.4) | 10 (16.9) |
| BMI (mean,STD) | 29.1 (3.9) | 27.7 (4.7) | 28.6 (4.3) | 28.9 (4.3) | 28.1 (3.8) |
| Statin use (N,%) | |||||
| Yes | 49 (66.2) | 50 (58.8) | 44 (50.6) | 50 (47.2) | 26 (44.1) |
| No | 25 (33.8) | 35 (41.2) | 43 (49.4) | 56 (52.8) | 33 (55.9) |
| Smoking status | |||||
| Ever | 28 (37.8) | 53 (62.4) | 50 (57.5) | 61 (57.5) | 41 (69.5) |
| Never | 46 (62.2) | 32 (37.6) | 37 (42.5) | 45 (42.5) | 18 (30.5) |
| Alcohol intake (mean g daily, STD) | 4.4 (7.8) | 8.1 (11.8) | 12.0 (14.9) | 11.3 (15.6) | 13.1 (21.5) |
| Hypertension | |||||
| Yes | 38 (51.4) | 40 (47.1) | 44 (50.6) | 57 (53.8) | 26 (44.1) |
| No | 36 (48.6) | 45 (52.9) | 43 (49.4) | 49 (46.2) | 33 (55.9) |
| Diabetes Mellitus | |||||
| Yes | 7 (8.2) | 13 (17.6) | 12 (13.8) | 19 (17.9) | 9 (15.3) |
| No | 78 (91.8) | 61 (82.4) | 75 (86.2) | 87 (82.1) | 50 (84.7) |
| Total energy intake, kcal/day (mean, STD)** | 2308 (1055) | 2310 (855) | 2180 (1,021) | 2,505 (983) | 2,533 (983) |
Excludes men with less than 6 months follow-up.
Over a median follow-up of 36 months (range 6–126 months), 76/411 patients (18.5%) experienced grade progression; and 12 patients died of other causes without documented progression. Median follow-up was 36 months (range 6–126) in men who did not progress, compared to 24 months (range 12–96 months) in those who did (P<0.01). Average consumption of other potential caffeine sources by progression status are listed in Supplementary Table 1.
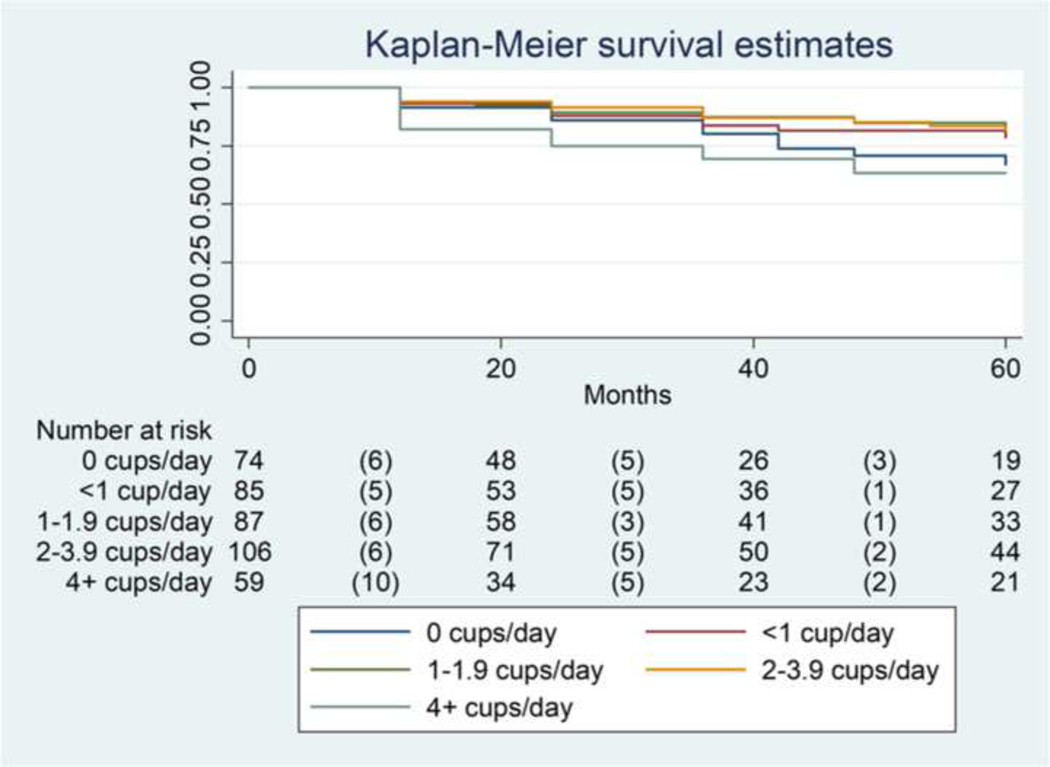
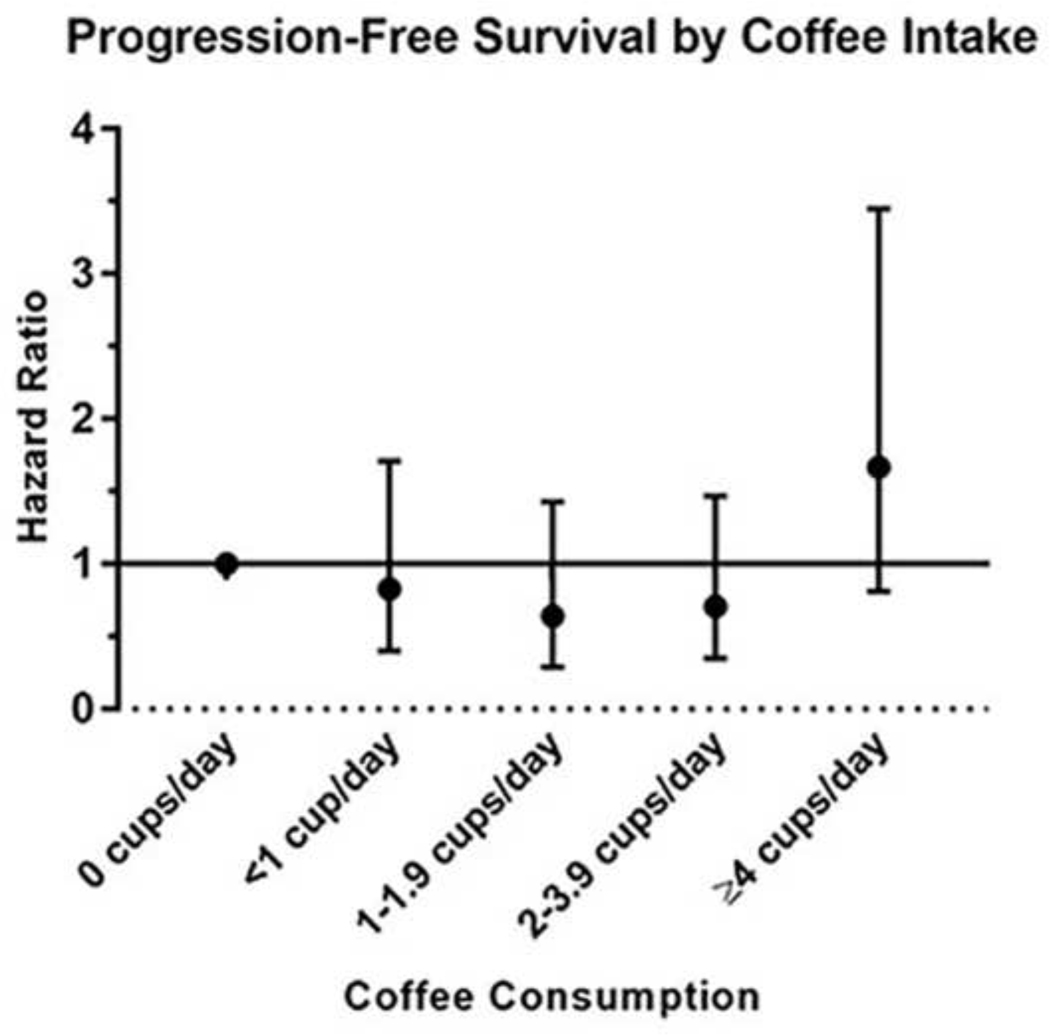
Five-year PFS rates by coffee intake are shown in Figure 1. Evaluation of the Log rank test revealed that consuming no coffee, or moderate amounts of coffee (1–4 cups per day) was not associated with PFS. However, consuming ≥4 cups per day was associated with shortened PFS (P=0.03). Notably, patients in this group had a high failure rate early in the follow-up time period (10/17 events [58.8%) within 20 months). In multivariable-adjusted models (Table 2) we similarly observed that associations of coffee intake and PFS followed a J-shaped relationship, P for non-linearity = 0.01 (Figure 2). While no association reached statistical significance, adjustment for a number of key confounders had little effect on the magnitude or shape of this unexpected relationship. Compared to non-consumers, associations with coffee intake up to <4 cups/day ranged from HR of 0.52 to 0.85 (all P>0.05), while intake of ≥4 cups/day appeared to be associated with worse outcomes (HR 1.67, 95% CI 0.81–3.45, P=0.16), when accounting for PSA, age and tumor length (Figure 2), as well as lifestyle and other factors (Table 2). Use of population specific quintiles, as well as additional adjustment for total energy intake in the clinical model revealed similar, although somewhat attenuated, results: (HR and 95% CI across energy-adjusted quintiles: 1.00 [ref], 0.62 [0.30–1.30], 0.80 [0.37–1.72], 0.73 [0.35–1.55], and 1.40 [0.70–2.80], respectively). Similar associations were observed for black (caffeinated) tea, regular soft drinks, and caffeine intake (Supplementary Table 2). With the exception of caffeine, other dietary components, as well as baseline testosterone level, were not strongly correlated with coffee intake (Supplementary Table 3). Exploratory stratified analyses evaluating the role of smoking, alcohol drinking status, BMI strata, statin use and baseline testosterone level exhibited consistent results and J-shaped trends across strata (data not shown).
Figure 1:
Five-year progression-free survival by coffee intake in prostate cancer patients on active surveillance
Parentheses indicate failure events
P=0.03 on Logrank test of 4+ cups/day vs. all others
P=0.17 for 0 cups/day vs. 1–4 cups
Table 2:
Association between coffee intake and progression-free survival in prostate cancer patients on active surveillance (n=411)
| Base Model* | Base + Clinical Characteristics** | Base + Clinical + Lifestyle*** | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
|
| |||||||||
| Coffee intake | |||||||||
| 0 cups/day | 1.00 | Ref | Ref | 1.00 | Ref | Ref | |||
| <1cup/day | 0.85 | 0.42–1.72 | 0.64 | 0.83 | 0.4–1.71 | 0.4 | 1.00 | Ref | Ref |
| 1–1.9 cup/day | 0.52 | 0.24–1.14 | 0.1 | 0.64 | 0.29–1.43 | 0.29 | 0.91 | 0.43–1.95 | 0.81 |
| 2–3.9 cups/day | 0.62 | 0.31–1.25 | 0.18 | 0.71 | 0.35–1.47 | 0.35 | 0.70 | 0.29–1.64 | 0.40 |
| ≥4 cups/day | 1.32 | 0.66–2.65 | 0.43 | 1.67 | 0.81–3.45 | 0.16 | 0.78 | 0.37–1.68 | 0.53 |
| Plinear trend | 0.75 | 0.28 | 1.92 | 0.89–4.14 | 0.03 | ||||
| Pnon-linear trend | 0.01 | 0.01 | 0.02 | ||||||
Base model adjusted for age.
Base + Clinical Characteristics model additionally includes PSA and composite tumor length.
Base + Clinical + Lifestyle additionally includes smoking, BMI, race, alcohol, and statin use
Figure 2.
Non-linear relationship between coffee intake and progression-free survival. Hazard ratios and 95% confidence intervals estimated in multivariable base + clinical model accounting for age, PSA, and composite tumor length; Pnonlinear trend =0.01.
In order to further investigate the role that may caffeine play in risk of progression among men with localized prostate cancer, we evaluated the effect of patient genotype at the rs762551 SNP. We evaluated associations of coffee intake and grade progression among a subset of 346 patients with available genotype data (Table 3). Among patients with an AA (fast metabolizer15) genotype, low to moderate coffee intake (up to 3 cups/day, as compared to 0 cups) was associated with significantly improved PFS (HR 0.36, 95% CI 0.15–0.88, P=0.03). Among patients with the AC/CC (moderate/slow) genotype, higher coffee intake appeared to be associated with increased risk.
Table 3:
Association between coffee intake and progression-free survival in prostate cancer patients on active surveillance by rs762551 genotype (n=411)
| rs762551 Genotype |
N | 0 cups/day N=59 |
0.1–2.9 cups/day N=208 |
≥3 cups/day N=79 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| AA “fast metabolizer” | 177 | 1.00 | Ref | Ref | 0.36 | 0.15–0.86 | 0.02 | 1.11 | 0.43–2.86 | 0.84 |
| AC/CC “moderate/slow metabolizer” | 169 | 1.00 | Ref | Ref | 1.71 | 0.40–7.41 | 0.47 | 3.34 | 0.63–17.62 | 0.16 |
Models adjusted for age, total caloric intake, PSA and tumor length
DISCUSSION
In a cohort of men with localized prostate cancer enrolled on a prospective AS protocol, we observed associations indicating that regular coffee consumption is likely safe, in moderation, for this population. Unique strengths of our study include robust clinical data regarding prostate cancer surveillance and grade progression in conjunction with dietary assessment. When taken with prior data indicating coffee is associated with lower risk of developing prostate cancer6 and improved outcomes in colorectal cancer patients11, our findings suggest low to moderate coffee consumption is likely safe. We cannot rule out potential increased risk of progression among heavy coffee drinkers and some sensitive groups, such as carriers of the slow caffeine metabolizer genotype.
While prior work has not examined the effect of coffee intake on time to grade progression in men on AS, multiple studies have demonstrated that coffee may have a protective effect in terms of prostate cancer risk. In a meta-analysis of 13 cohort studies, Liu et al. demonstrated that increased coffee intake may be associated with lower risk of prostate cancer, including risk of advanced disease.6 In the Health Professionals Follow-Up Study, coffee consumption was also associated with lower risk of prostate cancer and lethal disease.7 In a clinical cohort of colorectal cancer patients enrolled in the CALGB trial, Guercio et al. reported that increased coffee consumption was associated with improved disease-free and overall survival in patients who were found to have advance disease at the time of surgical resection.11 While this study evaluated a different outcome in a tumor that is clearly more aggressive than localized, low-volume prostate cancer, it provides credence to our findings that low to moderate coffee intake is likely safe. Coffee contains several plant-derived antioxidant and bioactive compounds, which may decrease subclinical levels of inflammation, insulin resistance, and insulin-like growth factor levels, all of which are relevant to prostate cancer patients in terms of overall health and progression risk.9,10
Our unexpected observation that ≥4 cups of coffee per day may confer increased risk in this group warrants further investigation in larger studies. Similar findings have also been reported for other malignancies. An umbrella review of the effect of coffee intake on cancer risk showed that increased consumption may be associated with increased incidence of lung cancer and bladder cancer in men.8 A prior study of prostate cancer in the SEER population of King County, Washington found that consumption of 2–6 cups of coffee per week was associated with more aggressive prostate cancer.17 These detrimental relationships may be driven by caffeine intake.
Perhaps most indicative of coffee and caffeine’s potential role in prostate cancer progression are our findings that “fast” caffeine metabolizers, as indicated by an AA genotype at rs762551, had pronounced protective effects of regular/caffeinated coffee intake, and mitigation of risk associated with very high consumption. Prior studies have demonstrated that fast caffeine metabolism may confer benefits in domains from myocardial infarction prevention18 to improved athletic performance15; however, our study offers novel evidence that genotype may have cancer-related implications, as well. Caffeine can effect cells through changes in DNA repair, apoptotic pathways, checkpoint integrity and p53 response19 and rats given high levels of caffeine have increased testosterone, epithelial cell proliferation, and prostatic androgen receptor expression.20 Notably, a recently published large population-based study (UK Biobank) reported inverse associations for coffee drinking (1 up to 8 or more cups per day) with mortality, regardless of caffeine metabolism genotype. 21 However, this characteristically different study among cancer-free individuals examined a different SNP related to CYP1A2 and is not directly comparable to our findings among patients on AS.
Despite the unique nature of our dietary assessment and clinical evaluation, the study is limited by both sample size/power and unmeasured potential confounders, such as physical activity. Importantly, consideration of factors known to be associated with prostate cancer and coffee intake, such as smoking22, diabetes and BMI23 did not materially change effects observed. However, the coffee query in our questionnaire only referred to regular, caffeinated coffee intake, limiting our ability to assess the effects of coffee and caffeine, independently. This weakness was mitigated somewhat through the use of caffeine metabolism-related genotype data and our assessment of other beverages. Finally, the present study relies on inherently limited patient reporting of usual coffee and other dietary intake at diagnosis.24
Our work is strengthened by the pairing of detailed clinical data with dietary assessment in a population in which the association between coffee intake and disease progression has not, to our knowledge, been previously investigated. Our finding that moderate coffee intake may be safe in men diagnosed with localized prostate cancer and enrolled on AS is important, though patients should be cautioned that it solely represents an association and may differ in some sensitive groups (e.g., slow caffeine metabolizers). Further prospective study, particularly with controlled or matched groups, is needed to determine if coffee intake is protective (and predictive) of prostate cancer progression. Until such evidence is available, our study can help assure clinicians and prostate cancer patients that they do not need to give up their daily cup (or two) of coffee.
Supplementary Material
ACKNOWLEDGEMENTS:
We would like to acknowledge all patients who participated in this study, without whom this investigation would not be possible. We also would like to thank Dr. Sara Strom for her early contributions to the study and dietary assessment. Finally, we would like to acknowledge the PRACTICAL consortium (http://practical.icr.ac.uk/blog/) and GAME-On OncoArray funding.
Research support:
NIH/NCI Cancer Center Support Grant P30 CA016672
List of Abbreviations
- AS
Active Surveillance
- BMI
Body Mass Index
- FFQ
Food Frequency Questionnaire
- GS
Gleason Score
- PFS
Progression Free Survival
- PSA
Prostate Specific Antigen
Footnotes
Conflict of interest: Dr. Davis is consultant for Intuitive and received scientific funding from Janssen and GenomeDX. All other authors have nothing to disclosure
REFERENCES
- 1.Cooperberg MR, Broering JM and Carroll PR: Risk Assessment for Prostate Cancer Metastasis and Mortality at the Time of Diagnosis. JNCI J. Natl. Cancer Inst. 2009; 101: 878–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Womble PR, Montie JE, Ye Z, et al. : Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur. Urol. 2015; 67: 44–50. [DOI] [PubMed] [Google Scholar]
- 3.Simpkin AJ, Tilling K, Martin RM, et al. : Systematic Review and Meta-analysis of Factors Determining Change to Radical Treatment in Active Surveillance for Localized Prostate Cancer. Eur. Urol. 2015; 67: 993–1005. [DOI] [PubMed] [Google Scholar]
- 4.Hamdy FC, Donovan JL, Lane JA, et al. : 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016; 375: 1415–1424. [DOI] [PubMed] [Google Scholar]
- 5.Loeb S, Bruinsma SM, Nicholson J, et al. : Active Surveillance for Prostate Cancer: A Systematic Review of Clinicopathologic Variables and Biomarkers for Risk Stratification. Eur. Urol. 2015; 67: 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H, Hu G-H, Wang X-C, et al. : Coffee consumption and prostate cancer risk: a meta-analysis of cohort studies. Nutr. Cancer 2015; 67: 392–400. [DOI] [PubMed] [Google Scholar]
- 7.Wilson KM, Kasperzyk JL, Rider JR, et al. : Coffee consumption and prostate cancer risk and progression in the Health Professionals Follow-up Study. J. Natl. Cancer Inst. 2011; 103: 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosso G, Godos J, Galvano F, et al. : Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017; 37: 131–156. [DOI] [PubMed] [Google Scholar]
- 9.Key TJ: Diet, insulin-like growth factor-1 and cancer risk. Proc. Nutr. Soc. 2011: 1–4. [DOI] [PubMed] [Google Scholar]
- 10.Kempf K, Herder C, Erlund I, et al. : Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am. J. Clin. Nutr. 2010; 91: 950–957. [DOI] [PubMed] [Google Scholar]
- 11.Guercio BJ, Sato K, Niedzwiecki D, et al. : Coffee Intake, Recurrence, and Mortality in Stage III Colon Cancer: Results From CALGB 89803 (Alliance). J. Clin. Oncol. 2015; 33: 3598–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis JW, Ward JF, Pettaway CA, et al. : Disease reclassification risk with stringent criteria and frequent monitoring in men with favourable-risk prostate cancer undergoing active surveillance. BJU Int. 2016; 118: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babaian RJ, Toi A, Kamoi K, et al. : A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J. Urol. 2000; 163: 152–157. [PubMed] [Google Scholar]
- 14.Palacios C, Daniel CR, Tirado-Gómez M, et al. : Dietary Patterns in Puerto Rican and Mexican-American Breast Cancer Survivors: A Pilot Study. J. Immigr. Minor. Health 2017; 19: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guest N, Corey P, Vescovi J, et al. : Caffeine, CYP1A2 Genotype, and Endurance Performance in Athletes. Med. Sci. Sports Exerc. 2018. [DOI] [PubMed] [Google Scholar]
- 16.Amos CI, Dennis J, Wang Z, et al. : The OncoArray Consortium: A Network for Understanding the Genetic Architecture of Common Cancers. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2017; 26: 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geybels MS, Neuhouser ML and Stanford JL: Associations of tea and coffee consumption with prostate cancer risk. Cancer Causes Control 2013; 24: 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Sohemy A, Cornelis MC, Kabagambe EK, et al. : Coffee, CYP1A2 genotype and risk of myocardial infarction. Genes Nutr. 2007; 2: 155–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito K, Nakazato T, Miyakawa Y, et al. : Caffeine induces G2/M arrest and apoptosis via a novel p53-dependent pathway in NB4 promyelocytic leukemia cells. J. Cell. Physiol. 2003; 196: 276–283. [DOI] [PubMed] [Google Scholar]
- 20.Sarobo C, Lacorte LM, Martins M, et al. : Chronic caffeine intake increases androgenic stimuli, epithelial cell proliferation and hyperplasia in rat ventral prostate. Int. J. Exp. Pathol. 2012; 93: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loftfield E, Cornelis MC, Caporaso N, et al. : Association of Coffee Drinking With Mortality by Genetic Variation in Caffeine Metabolism: Findings From the UK Biobank. JAMA Intern. Med. 2018. Available at: http://archinte.jamanetwork.com/article.aspx?doi=10.1001/jamainternmed.2018.2425, accessed July 21, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Nunzio C, Andriole GL, Thompson IM, et al. : Smoking and Prostate Cancer: A Systematic Review. Eur. Urol. Focus 2015; 1: 28–38. [DOI] [PubMed] [Google Scholar]
- 23.Anon: World Cancer Research Fund International/American Institute for Cancer Research Continuous Update Project Report: Diet, Nutrition, Physical Activity, and Prostate Cancer. 2014. Available at: www.wcrf.org/sites/default/files/Prostate-Cancer-2014-Report.pdf.
- 24.Subar AF, Kipnis V, Troiano RP, et al. : Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am. J. Epidemiol. 2003; 158: 1–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.