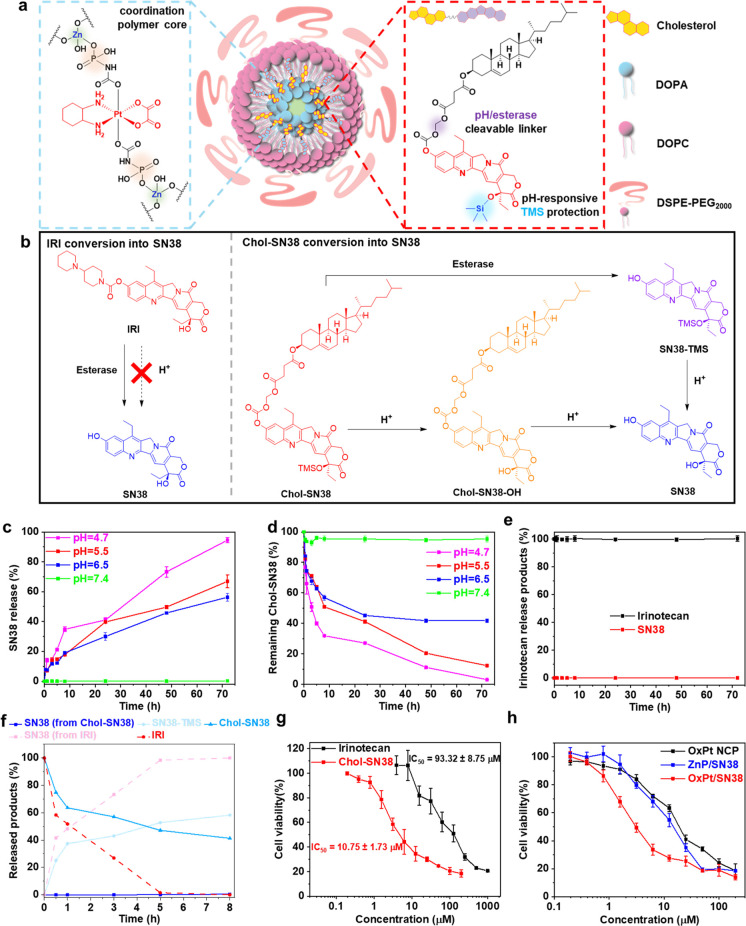
Figure 1.
OxPt/SN38 structure, drug release, and cytotoxicity. (a) Schematic illustration of OxPt/SN38 particles with a Zn-(OxPt-bisphosphate) coordination polymer core and a shell of Chol-SN38, cholesterol, DOPC, and DSPE-PEG2000. In Chol-SN38, SN38 was doubly modified by conjugation to cholesterol via a cleavable acetal linkage at the phenol position and TMS protection at the 20-hydroxy position. (b) Mechanisms for SN38 conversion from IRI and Chol-SN38. SN38 release (c) and remaining Chol-SN38 (d) from OxPt/SN38 in pH = 4.7, 5.5, 6.5, and 7.4 PBS at 37 °C over 72 h. (e) IRI product conversion in pH = 4.7 PBS at 37 °C over 72 h. (f) Conversion of IRI or Chol-SN38 in OxPt/SN38 to SN38 in esterase-containing PBS (pH = 7.4). (g) MTS assays of IRI and Chol-SN38 on MC38 cells. (h) MTS assays of OxPt NCP, ZnP/SN38, and OxPt/SN38 on MC38 cells.