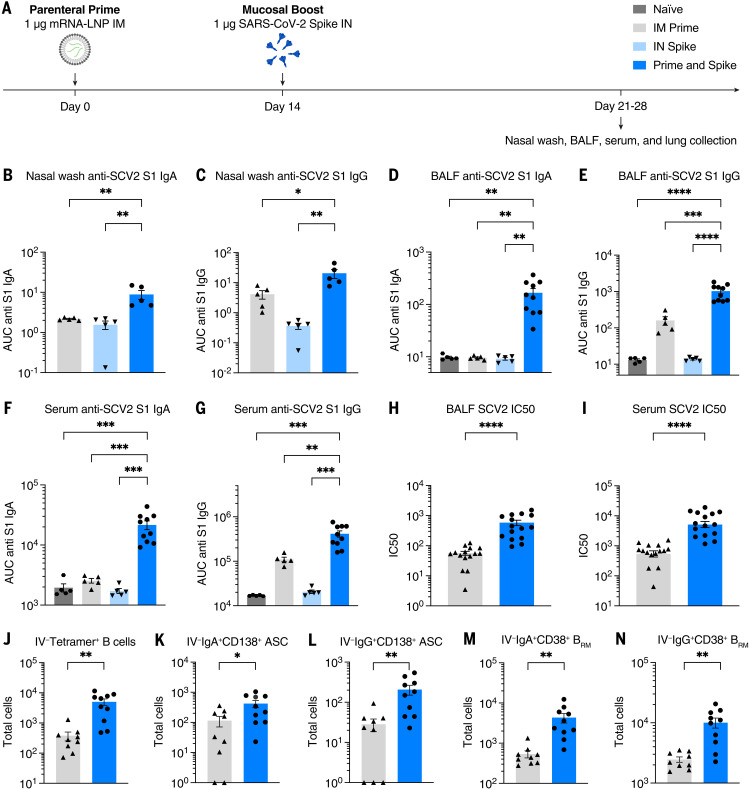
Fig. 1. IN boosting with stabilized SARS-CoV-2 spike induces mucosal humoral memory.
(A) Experimental schema. Mice were intramuscularly immunized with 1 μg of mRNA-LNPs encoding full-length SARS-CoV-2 (SCV2) spike protein (Pfizer/BioNTech BNT162b2), followed by IN immunization with 1 μg of prefusion-stabilized (Hexapro), trimeric, recombinant SCV2 spike protein 14 days after mRNA-LNP immunization. Fourteen days after IN boost, serum, BALF, and nasal washes were collected to assess binding and neutralizing antibody responses. Lung tissues were collected for extravascular B cell analysis. (B to G) Measurement of SCV2 spike S1 subunit–specific (B) nasal wash IgA, (C) nasal wash IgG, (D) BALF IgA, (E) BALF IgG, (F) serum IgA, and (G) serum IgG in naïve mice, mice immunized with mRNA-LNP IM (IM Prime), mice immunized with the spike protein IN (IN Spike), or mice IM primed and IN boosted with spike (P&S). (H to K) Measurement of neutralization titer against SCV2 spike–pseudotyped vesicular stomatitis virus (VSV) in (H) BALF and (I) serum. (J to N) Using CD45 IV labeling, various extravascular (IV labeling antibody negative) B cell subsets were measured, including RBD tetramer-binding B cells, IgA+ BRM cells, IgG+ BRM cells, IgA+ ASCs, and IgG+ ASC in lung tissues from IM Prime or P&S mice. Mean ± SEM. Statistical significance was calculated by means of [(B) to (G)] one-way analysis of variance (ANOVA) or [(H) to (N)] Student’s t test; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Individual data points are represented and are pooled from two or three independent experiments.