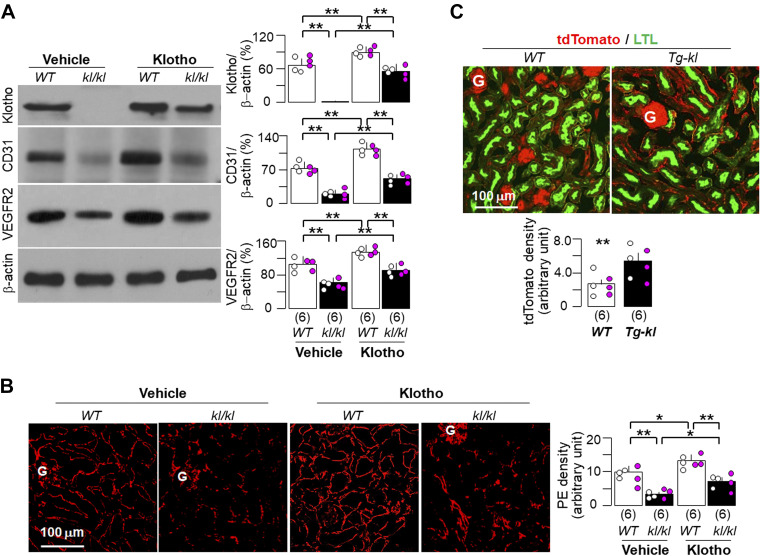
Figure 3.
Klotho improved peritubular capillaries in Klotho-deficient mice. Twelve wild-type (WT) or kl/kl mice at 4 wk of age were fed with normal rodent chow and randomly assigned into two treatments (vehicle and recombinant Klotho protein) through intraperitoneal implantation of osmotic minipumps for a total duration of 4 wk. Mice were euthanized, and the kidneys were harvested for further experiments (A and B) after 4 wk of implantation. A: immunoblot analysis for endothelial marker (CD31), Klotho, vascular endothelial growth factor receptor type 2 (VEGFR2), and β-actin in total kidney lysates. A, left: representative immunoblots. A, right: quantitative analysis of all immunoblots from the four groups. B: immunofluorescent images for endothelial marker (PE; red) in kidney sections. B, left: representative immunofluorescent images. Scale bar = 100 µm. B, right: quantitative analysis of PE staining density on kidney sections with ImageJ software. C: WT and Tg-kl mice harboring tdTomato and Tie2-Cre genes were euthanized at 12 wk, and the kidneys were harvested to image the vasculature. C, top: representative immunofluorescent images for tdTomato signal (red) and Lotus tetragonolobus lectin (proximal tubular marker; green). Scale bar = 100 µm. C, bottom: quantitative analysis of tdTomato staining density on kidney sections with ImageJ software. A–C: quantitative data are expressed with scatterplots of individual data points (open circles indicate male mice and pink circles indicate female mice) and means ± SD (bars and errors) from each group. Statistical significance was evaluated by two-way ANOVA followed by a Student–Newman–Keuls test. Significant differences were accepted when *P < 0.05 and **P < 0.01 between two groups. The sample number in each group is presented in parentheses underneath each corresponding bar. G, glomerulus; PE, a cocktail of primary antibodies against platelet-endothelial cell adhesion molecule-1 and endomucin.