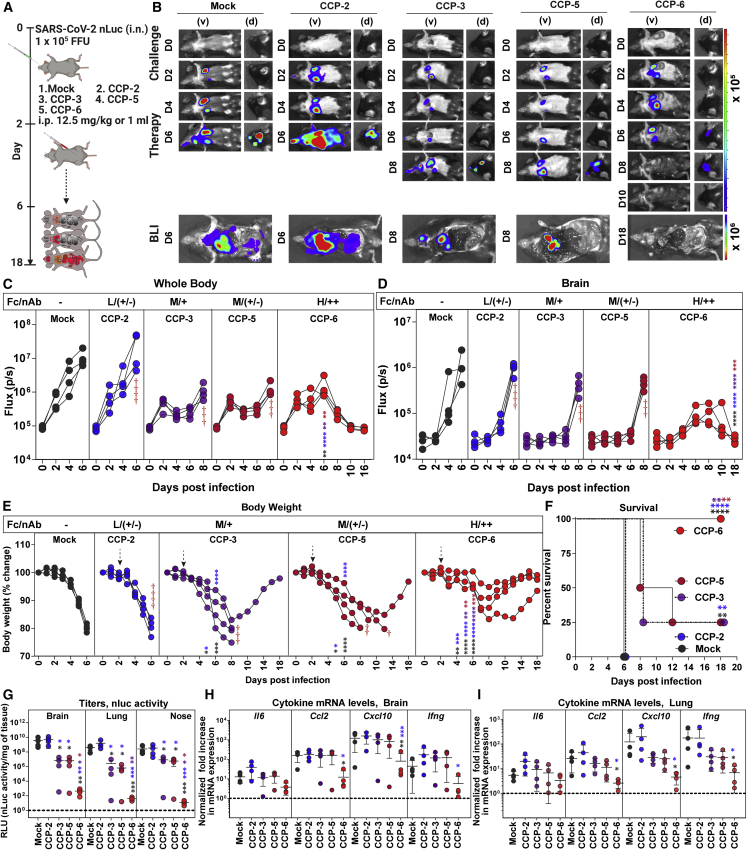
Figure 2.
In vivo efficacies of selected CCPs in K18-hACE2 mice against lethal SARS-CoV-2 challenge during therapy
(A) Experimental design for screening in vivo efficacy of indicated CCPs delivered under therapy (+2 dpi, i.p.) in K18-hACE2 mice challenged with 1 × 105 FFU WA1 SARS-CoV-2-nLuc (i.n.). hIgG1-treated mice were used as control (Mock).
(B) Representative BLI images of SARS-CoV-2-nLuc-infected mice in ventral (v) and dorsal (d) positions for experiment as in (A). Scale bars denote radiance (photons/s/cm2/steradian).
(C and D) Temporal quantification of nLuc signal as flux (photons/s) computed non-invasively in indicated tissues.
(E) Temporal changes in mouse body weight with initial body weight set to 100% for experiment. Cross symbol, death.
(F) Kaplan-Meier survival curves of mice (n = 4 per group) statistically compared by log-rank (Mantel-Cox) test.
(G) Viral loads (nLuc activity/mg) in indicated tissue measured after necropsy on Vero E6 cells as targets. Undetectable virus amounts were set to 1.
(H and I) Fold change in indicated cytokine mRNA expression in brain and lung tissues. The data were normalized to Gapdh mRNA expression in the same sample and that in non-infected mice after necropsy. CCP classification for associated %ADCC (Fc) are shown as low (L), Moderate (M), and High (H). Relative nAb titer of CCPs (ID50 < 1:250) are shown as ++, +, and +/−. Each curve in (C–E) represents an individual mouse. Data in (C–I) are from two independent experiments and n = 2 mice per group. Grouped data in (C–E) and (G–I) were analyzed by 2-way ANOVA followed by Tukey’s multiple comparison tests. Statistical significance for group comparisons to mock controls are shown in black, with convalescent plasma CCP-2 shown in blue, with CCP-3 shown in purple, and CCP-5 are shown in light red. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001; mean values ± SD are depicted. See also Figure S1