Abstract
Objective
This study aimed to determine if delayed cord clamping (DCC) affected brain myelin water volume fraction mineral (VFm) value and neurodevelopment in term infants.
Study Design
This was a single-blinded randomized controlled trial of healthy pregnant women with term singleton fetuses randomized at birth to either immediate cord clamping (ICC) (≤ 20 seconds) or DCC (≥ 5 minutes). Follow-up at 12 months of age consisted of blood work for serum iron indices and lead levels, a nonsedated magnetic resonance imaging (MRI), followed within the week by neurodevelopmental testing.
Results
At birth, 73 women were randomized into one of two groups: ICC (the usual practice) or DCC (the intervention). At 12 months, among 58 active participants, 41 (80%) had usable MRIs. There were no differences between the two groups on maternal or infant demographic variables. At 12 months, infants who had DCC had increased white matter brain growth in regions localized within the right and left internal capsules, the right parietal, occipital, and prefrontal cortex. Gender exerted no difference on any variables. Developmental testing (Mullen Scales of Early Learning, nonverbal, and verbal composite scores) was not significantly different between the two groups.
Conclusion
At 12 months of age, infants who received DCC had greater myelin content in important brain regions involved in motor function, visual/spatial, and sensory processing. A placental transfusion at birth appeared to increase myelin content in the early developing brain.
Keywords: umbilical cord, iron, placental transfusion, delayed cord clamping, myelin
In the first few minutes after birth, a delay in clamping the umbilical cord or milking the cord results in the return of a large volume of blood from the placenta to the infant.1,2 The expanded blood volume, used for oxygenation and other essential functions in utero, enhances lung perfusion and successful transition to breathing and oxygenation. This placental transfusion also facilitates transition by increasing blood flow to the circulatory beds while the infant’s various organs (i.e., lung, liver, kidney) assume many functions previously maintained by the placenta.3–8
A placental transfusion provides up to 50% increase in iron-rich red blood cells (RBCs) for the newly born infant as they hold approximately 70 to 80% of the iron in the human body.2 Ferritin, a measure of stored body iron, is significantly higher in infants at 4 to 6 months of age who receive delayed cord clamping (DCC) compared with those with immediate cord clamping (ICC).9–11 This is important because an adequate iron supply is critical for healthy and normative development of brain structures and neurotransmitter systems.12–15 The increase in RBCs obtained due to DCC may provide a critical early iron endowment for oligodendrocytes, the most metabolically active cells in the brain. Iron is critical to the maturation and functioning of the oligodendrocytes responsible for brain myelination,12,13 which is essential for establishing normative brain connectivity and cognitive function. As seen in previous histological and developmental neuroimaging studies, myelination progresses rapidly during the first years of life and may be most sensitive during this early postnatal period.12–15 Animal and human studies have documented that iron deficiency (ID) in early infancy negatively impacts cognitive, motor, social-emotional, and behavioral development.16,17 New evidence links ID in infancy with neuropsychiatric diseases in adults raising further concerns about the practice of ICC.18
We previously showed a hematologic advantage following a 5-minute delay in cord clamping at 48 hours (hemoglobin) and 4 months of age (ferritin), without increased morbidity.11,19 Using a quantitative magnetic resonance imaging (MRI) technique, mcDESPOT,20 we found a positive correlation between ferritin levels and brain myelin content. Infants randomized to the DCC group had increased white matter brain growth in the internal capsule and other early maturing brain regions associated with motor, visual, and sensory processing/function at 4 months of age.11 This is a report of the results of DCC at 12 months of age on brain myelin water volume fraction (VFm) and neurodevelopment in our cohort of term infants. Our hypothesis was that infants with DCC would have greater myelin volume at 12 months of age than infants who had ICC.
Methods and Materials
Study Design
This was a single center, blinded randomized controlled trial conducted at Women and Infants Hospital and Brown University, both in Providence, Rhode Island. All Institutional Review Boards approved the study including that of the University of Rhode Island. The study was registered with ClinicalTrials.gov—NCT 01620008. The study outcomes from the birth, 2 day, and 4 month results have been fully described previously.11,19 Briefly, healthy pregnant women (with term singleton fetuses) were eligible if they were ≥18 years old and willing to participate in the follow-up. Following written informed consent and assent by the obstetrical (OB) provider, 73 infants were randomized at birth to either ICC (≤ 20 seconds) or DCC (≥ 5 minutes) in the time frame between July 2012 and November 2015. Follow-up of these infants was conducted at both four and 12 months of age. At 12 months of age, follow-up consisted of serum iron indices and lead levels, a nonsedated MRI, followed within the week by neurodevelopmental testing. This was conducted between July 2013 through November 2016.
Intervention, Randomization, and Blinding
Just before birth, blocked stratified randomization was used (in sequenced and sealed envelopes) to assign women to either ICC (< 20 seconds) or DCC (≥ 5 minutes). The research assistant informed the OB team of the group assignment. If the OB provider thought that the clinical situation did not allow for DCC, the umbilical cord was milked five times before cord clamping (n = 8). Infants were placed skin-to-skin for at least 30 minutes and infant care was directed by the nursing and pediatric staff. It was not possible to blind the OB staff to the group assignment. However, all laboratory personnel and those who conducted follow-up were blinded to group assignment.
Participant Follow-Up
At 12-months, a blood sample was collected by a trained phlebotomist for a complete blood count, lead level, iron indices (ferritin, transferrin, and soluble transferrin receptor [sTfR]), and C-reactive protein or CRP (Supplementary Material, available in the online version).
For the 12-month MRI scan, infants (sleeping/unsedated) were placed in a Siemens Tim Trio 3 Tesla scanner. Brain myelin content was assessed using the mcDESPOT technique by the VFm, a quantitative MRI method that models the acquired MRI signal into three specific tissue compartments (i.e., myelin, intraextracellular, and free) and quantifies the relaxation properties of each of these water compartments. The volume fraction associated with the myelin water compartment, VFm, is a proxy measure of myelin content. This method has previously been used to investigate patterns of brain development during childhood20–22 as well as examine alterations of myelin content in neurodevelopmental23 and neurodegenerative disorders.24–26 Additional details regarding the MRI protocol can be found in Supplementary Material (available in the online version).
The developmental assessment was completed by a specialist at the MRI center within seven days of a successful MRI. The two assessment tools were the Mullen Scales of Early Learning (MSEL)20,27 and Brief Infant Toddler Social Emotional Assessment (BITSEA).28 The MSEL (birth—68 months) is composed of five scales which include gross and fine motor, visual reception, and expressive and receptive language and is used to assess cognitive ability and motor development. It is straightforward to administer and is highly reliable. Scoring is reported by scales (verbal and nonverbal) and is used as an early learning composite score. The BITSEA, a parent completed questionnaire, is used to screen for social-emotional and behavioral competence resulting in two scales: the Problem scale and the Competence scale. The Problem scale assesses problems and delays in competence such as externalizing and internalizing problems, problems of dysregulation, maladaptive behaviors, and atypical behaviors. The Competence scale assesses social-emotional abilities such as sustained attention, compliance, mastery motivation, prosocial peer relations, empathy, imitation/play skills, and social relatedness.
Sample Size
As no previous data exist for the effects of umbilical cord clamping time on VFm, we estimated the standard deviation of VFm estimates in white matter to be 5% in healthy children.29 To reliably measure a 5% VFm difference between the control and experimental groups, using a two-sample t-test (α = 0.05, power = 0.80), 16 observations per group were required.
Power and Statistical Analyses
Data analyses included two-sided Pearson tests, two-sample t-tests, and Wilcoxon rank-sum tests for non-normally distributed variables. Primary analyses were conducted using intention to treat, and sensitivity analyses were conducted using actual treatment to assess the robustness of the findings and to examine results of the biological variables. Log transformation was used for the analysis of the ferritin levels due to non-normal distribution of the ferritin data. The level of significance was 0.05 (two-tailed) for the main effects. Data were analyzed with SAS 9.3 (SAS Institute, Inc, Cary, NC) and SPSS Version 23 (IBM Corp, Armonk, NY).
Image Analysis and Statistical Testing
VFm differences between children in the DCC and ICC groups were investigated using voxel-wise unpaired t-tests. For all voxel-wise analyses, the FMRIB Software Library package (FMRIB Analysis Group, Oxford, United Kingdom) was used to nonparametrically evaluate the statistical models using permutation testing (randomize) and 5,000 permutations. Significance was defined as p < 0.05, with correction for the multiple comparisons in MRI data using a cluster-based technique.30,31
Results
At the time of birth, 73 healthy term women were randomized into one of two groups: ICC (<20 seconds) or DCC (≥ 5 minutes) (Fig. 1). No data from the preference group were used for this paper (refer to note in Fig. 1). At 12 months, 58 (79%) infants were active study participants. Of those, 52 infants (90%) had a 12-month blood draw and 51 infants (88%) underwent an MRI scan. Of the 51 attempted MRI scans, 41 (80%) were usable. The children with usable MRIs were more likely to be from families who had private insurance and listed race as white. All the data reported here are from the 41 infants with a successful MRI scan (21 in DCC group, 20 in ICC group) noted as the “randomized MRI cohort.”
Fig. 1.
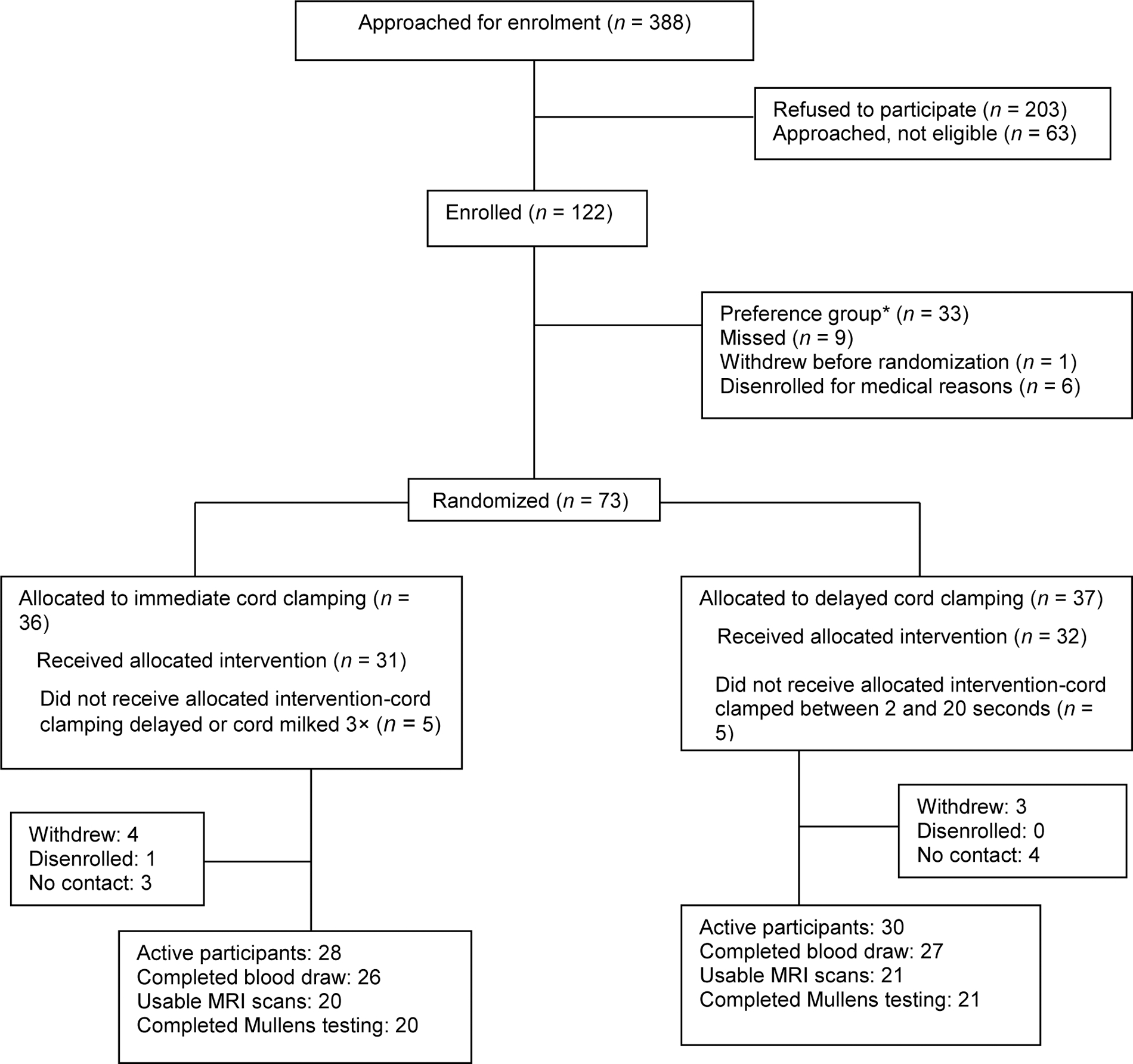
Infant brain study randomized ITT at 12M. The preference group was established because many mothers did not want to be randomized. We hoped that we would get equal numbers of those wanting DCC and those wanting to harvest cord blood. However, 32 to 1 wanted DCC versus harvesting so we did not have equal cohorts to compare. No data from the preference group is included in this paper in any manner. DCC, delayed cord clamping; ITT, intention to treat.
Table 1 shows no significant differences between the two groups on maternal or infant demographic variables. Infants from the 12-month MRI randomized cohort who received DCC had a longer cord clamping time (per protocol), less residual placental blood volume (RPBV), and higher 48-hour hemoglobin and hematocrit levels. There were no differences at birth in the umbilical cord hemoglobin and ferritin level between the groups (data not shown). At 4 months there were no differences in hemoglobin and hematocrit levels but there were significant differences in ferritin and log ferritin levels, favoring the DCC group (Table 1). There was no difference in the proportion of successful scans between the ICC and DCC groups.
Table 1.
Maternal and infant demographics and clinical variables at birth and 4 mo for the 12-mo MRI cohort ITT
| Maternal characteristics | DCC (n = 21) | ICC (n = 20) | p-Value |
|---|---|---|---|
| Age (y) | 30 ± 6 | 28 ± 6 | 0.32 |
| Race, white | 15 (71) | 14 (70) | 0.92 |
| Primipara | 9 (43) | 9 (45) | 0.89 |
| Years of education | 15 ± 3 | 14 ± 3 | 0.49 |
| Public insurance | 10 (48) | 10 (50) | 0.88 |
| Ferritin at admission (ng/mL) | 26 ± 27 | 18 ± 15 | 0.20 |
| Vaginal birth | 15 (71) | 13 (65) | 0.66 |
| Infant | |||
| Birthweight (g) | 3,507 ± 511 | 3,321 ± 394 | 0.20 |
| Male: Female | 12:9 | 13:7 | 0.61 |
| Cord clamping time (s), includes UCM | 42 (5–647) | 10 (3–360) | 0.001b |
| without UCMa | 306 (55–647) | 9 (3–21) | 0.0001b |
| Residual placental blood volume (mL/kg) | 21 ± 9 | 32 ± 11 | 0.002b |
| Two-day hemoglobin (g/dL) | 19.6 ± 1.9 | 17.6 ± 2.0 | 0.003b |
| Two-day hematocrit (%) | 59 ± 6 | 52 ± 5 | 0.001b |
| Four-month hemoglobin (g/dL) | 11.8 ± 0.8 | 11.7 ± 0.7 | 0.65 |
| Four-month hematocrit (%) | 34 ± 2 | 34 ± 2 | 0.93 |
| Four-month ferritin (ng/mL) | 91 ± 57 | 61 ± 30 | 0.04a |
| Four-month logferritin | 4.4 ± 0.5 | 4.0 ± 0.5 | 0.02a |
Abbreviations: DCC, delayed cord clamping; ICC, immediate cord clamping; ITT, intention-to-treat; SD, standard deviation; UCM, umbilical cord milking.
Note: N (%), mean ± SD or median (full range).
p < 0.05.
p < 0.001.
Table 2 shows the 12-month infant clinical variables for this cohort. At 12 months of age, there were no differences in ferritin levels between groups, other iron indices, or CRP (data not shown). Infants with DCC had slightly lower hematocrit and hemoglobin levels. None of the mothers reported that infants received iron supplementation, but all infants were eating solid food by 12 months of age (data not shown). There was no significant difference between groups on breast or bottle feeding in the first 4 months (data not shown). The MSEL early learning, nonverbal, and verbal composite scores were not significantly different between the two groups (Table 2).
Table 2.
Clinical 12-mo variables for MRI cohort ITT
| DCC (n = 21) | ICC (n = 20) | p-Value | |
|---|---|---|---|
| Hemoglobin (g/L) | 11.5 ± 0.8 | 12.1 ± 0.7 | 0.02a |
| Hematocrit (%) | 35 ± 3 | 37 ± 2 | 0.03a |
| Ferritin (ng/mL) | 35 ± 21 | 49 ± 59 | 0.77 |
| Logferritin | 3.4 ± 0.6 | 3.5 ± 0.8 | 0.53 |
| Mean cell volume (fL) | 78 ± 4 | 79 ± 3 | 0.51 |
| Transferrin (mg/dL) | 257 ± 44 | 253 ± 31 | 0.76 |
| sTfR (mg/L) | 4.5 ± 0.9 | 4.5 ± 0.8 | 0.92 |
| CRP (mg/L) | 0.3 (0.2–3.2) | 0.5 (0.2–2.0) | 0.22 |
| Lead (mcg/dL) | 1 (0–5) | 2 (0–3) | 0.15 |
| Mullens early learning composite | 100 ± 14 (n = 20) | 102 ± 11 | 0.62 |
| Mullens verbal composite | 100 ± 21 (n = 20) | 103 ± 16 | 0.54 |
| Mullens nonverbal composite | 112 ± 11 | 113 ± 10 | 0.83 |
| BITSEA problem score | 7 ± 5 | 7 ± 4 | 0.97 |
| BITSEA competence score | 17 ± 3 | 15 ± 4 | 0.13 |
Abbreviations: BITSEA, brief infant toddler social emotional assessment; CRP, C-reactive protein; DCC, delayed cord clamping; ICC, immediate cord clamping; ITT, intention to treat; MRI, magnetic resonance imaging; SD, standard deviation; sTfR, soluble transferrin receptor.
Note: Mean ± SD or median (full range).
p < 0.05.
Analyses of the VFm differences revealed that infants with DCC had higher VFm in regions localized within the right and left internal capsules, the right parietal and occipital white matter as well as right hemispheric white matter of the prefrontal cortex (Fig. 2).
Fig. 2.
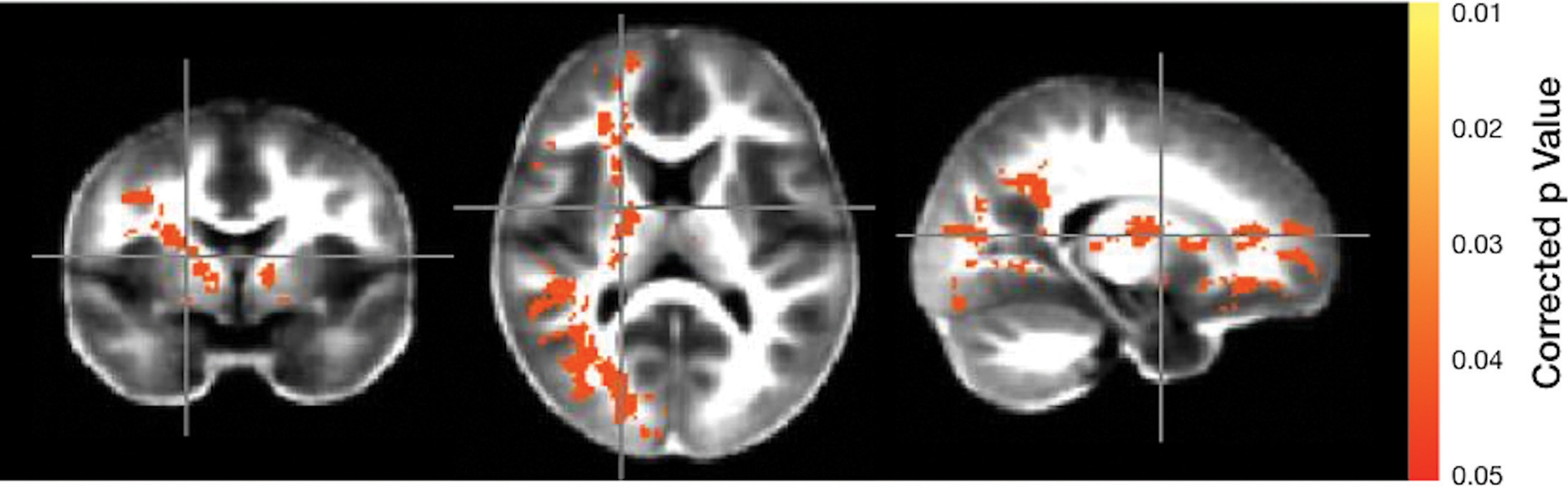
MRI view comparison of brain myelin. Significance is indicated by the color scale on the right with yellow at a p-value of 0.01 and red at p-value of 0.05. The color represents areas in which myelin is greater in infants who had DCC compared with those who had ICC. DCC, delayed cord clamping; ICC, immediate cord clamping; MRI, magnetic resonance imaging.Q12
Although the groups were stratified by sex, this did not exert any effect on the bloodwork, MRI, or developmental testing (data not shown).
Discussion
This is the first study to examine the timing of umbilical cord clamping and its impact on myelin content in the early developing infant brain at 12 months. Waiting at least 5 minutes before clamping the umbilical cord at birth appears to result in a sustained increase of myelin content at 12 months in brain regions involving motor pathways and areas responsible for visual/spatial and sensory processing. The right and left internal capsules contain vital communication pathways while the right parietal region encompasses motor pathways. The right occipital region is reported to oversee visual spatial processing. The right frontal and orbital pathways are responsible for executive function and inhibition.
Previously, we showed that higher ferritin levels were associated with increased brain myelin content and that DCC resulted in children having higher myelin content than ICC children in developing white matter regions by 4 months of age.11 In the current study, we extend these findings and show that DCC children continue to exhibit higher myelin content than ICC children at 12 months of age. The overlap between the spatial extent of our previous findings with the current findings suggests that the increased myelin content in DCC children is sustained from 4 to 12 months. However, we also observe additional brain regions, such as occipital and prefrontal white matter regions, to have higher myelin content in DCC children that were previously not observed at 4 months of age. This may indicate that DCC and the increased in iron stores resulting from DCC, alter the neurodevelopmental trajectory of myelination, particularly as these brain regions are known to develop at later stages of development.13 Continued follow-up of this existing cohort and longitudinal analyses will allow us to investigate this hypothesis.
One probable mechanism for the increase in myelin content at 12 months is related to the increased availability of iron for early myelinogenesis in the infants exposed to a 5-minute delay in cord clamping. It is well known that iron is essential for the maturation and function of oligodendrocytes.12,17 During the critical period of early infancy, the developing brain is dependent on adequate iron and iron stores to support the demands of myelin growth which sharply accelerates during the first few months after birth.32 In this study, higher ferritin levels at 4 months of age appeared to support increased brain myelin in important brain regions and we now show this extends out to 12 months of age. The practice of cutting the cord quickly at birth prevents an infant’s access to this essential iron source and may result in ID, placing them at risk for reduced brain myelin content. ID can result in epigenetic changes via both deoxyribonucleic acid methylation and histone modification pathways potentially leading to the negative long-term neurodevelopmental impact seen in children with ID in infancy.16,33
Infants in this study who received DCC left less blood (34%) behind in the placenta compared to those who had ICC. This confirms a placental transfusion occurred with DCC. Along with RBCs, this blood contains a large volume of stems cells and essential plasma.34 Although we did not measure stem cells, others have shown that RPBV contains a large number and variety of naïve stem cells including preoligodendrocytes.35,36
Ferritin has been an important biomarker in multiple DCC clinical trials for both term and preterm infants. However, by 12 months of age, ferritin levels usually do not significantly differ between children exposed to ICC or DCC.37,38 This provides support for our hypothesis that better iron stores at 4 months of age (as a result of placental transfusion) result in more myelin content in the early developing brain regions at both at 4 and 12 months of age.
In this cohort of children, there was a significant difference in cord clamping time at birth. The ideal time for clamping the cord remains controversial. A 5-minute delay was chosen to reflect a clearer contrast between our two groups (ICC and DCC) and to offset any potential effect from infants being placed on the maternal abdomen (i.e., infant held above the level of the placenta). Examining varying cord clamping times with infants placed skin-to-skin, we obtained differences of 35 mL/kg RPBV with ICC, 25 mL/kg with a 2-minute delay, 11 mL/kg with a 5-minute delay, and 18 mL/kg with cord milking (five times). This suggests when infants are placed on the maternal abdomen, more time is needed to achieve an adequate placental transfusion.39
Two studies recently have added new information characterizing intact cord blood flow that supports physiologic cord clamping and extended delay. Boere et al measured blood flow in the umbilical cord after birth and before cord clamping with Doppler ultrasound in 30 uncomplicated term spontaneous vaginal births.40 Over 80% of the infants in the study had umbilical arterial flow (UAF) continued for at least 4.22 minutes after birth and by 6 minutes, 43% still had UAF when the cord was clamped. The UAF was pulsatile similar to the infant’s heartbeat and flow was mainly unidirectional, from the infant to the placenta. In another study of the duration of cord pulsations by palpation in 102 infants with DCC at vaginal birth, pulsations continued for 213 seconds median duration.41 When dividing the group in half below and above the median, they found that infants with longer duration of cord pulsations had higher birthweights (3,530 vs. 3,250, p = 0.005) and longer third stage of labor (12 vs. 8 minutes, p < 0.001), without an increased risk of postpartum hemorrhage. An observational study of 900 Swedish women and infants with extended cord clamping time (median 6 minutes, ranging from 0 to 23.5 minutes), reported less postpartum hemorrhage and no difference in infant bilirubin levels with longer cord clamping times.42 Extending cord clamping times (>180 seconds) is a very suitable area for further investigation.
The children in this cohort showed no significant differences in the MSEL verbal or nonverbal language scores or in overall cognitive abilities or social emotional competence on the BITSEA. This was not an unexpected finding and is similar to the results reported by Andersson et al in over 300 Swedish healthy term infants randomized to either ICC (≤ 10 seconds) or DCC (≥ 180 seconds) at birth.38 Andersson et al found at 12 months of age, DCC showed no effect on developmental outcomes. However, at 4 years, they found higher fine motor skills and social domain scores in the DCC group, especially in boys. It is likely that developmental differences may not be discernable until a child needs to use higher order functioning such as executive function.15 Interestingly, Kc et al, using the Ages and Stages Questionnaire, reported higher scores in the infants who received DCC (≥ 180 seconds) at 12 months in a study of more than 330 Nepalese term infants.43 The children who received DCC also had higher ferritin levels at 8 months of age suggesting that the practice of waiting to clamp the cord may have a greater impact in less developed countries.
In spite of employing many varied techniques for retention, our rate did not reach our expectations. At 12 months, there were 58 (79%) active participants in the study. However, only 41 were able to complete the triad of laboratory work, MRI (successful), and developmental testing. Twelve months of age appears to be a difficult time to obtain MRIs. Our rate of 80% usable scans was much lower than the 4-month rate (92%). In addition, as the developmental testing was planned to coincide with the MRI scans, an unsuccessful MRI meant developmental testing was not completed. MRIs were done at nap time or in the evening and depended on the child’s ability to fall (and stay) asleep in a strange environment. Parents often spent 3 to 5 hours at the MRI facility and several made more than one attempt to obtain successful imaging. These factors may have been more stressful for parents with fewer resources resulting in poorer retention of low-income families in this study.
We used multiple retention strategies to keep participants actively engaged. These included mailing information before the visits; phone contacts, text, or email by the research nurse and the MRI coordinator; ongoing contact with the research assistant who had attended the birth; and increased monetary incentive for the MRI and developmental testing. A study newsletter was sent twice a year. Celebration cards were sent on birthdays and important nonreligious holidays. Most references describing excellent follow-up rates report working with people who had high subject motivation to return due to serious health conditions. Follow-up of preterm infants or infants with significant health concerns have higher follow-up rates as compared with healthy preterm infants (personal communication: Betty R. Vohr, January 2018). A majority of the mothers who did not continue in our study at 12-months reported that their busy lifestyles such as work outside the home, care of other children, or a subsequent pregnancy interfered with their commitment to the study. None of the mothers reported any dissatisfaction with the study protocol or with study personnel.
The current findings complement and build upon our previous findings that myelin content is influenced by DCC. Additionally, they suggest that the mcDESPOT derived VFm measurements provide regionally specific estimates of myelin content and can track longitudinal changes. Indeed, VFm measurements qualitatively agree with histological patterns of myelin, while studies utilizing mcDESPOT in early childhood highlight the sensitivity of VFm to white matter maturation,20–22,44 Moreover, mcDESPOT has been shown to provide stable VFm estimations.45 Still, future studies are necessary to quantitatively validate mcDESPOT measurements.
While we have focused on myelin content being one aspect of brain development influenced by DCC, due to the role that iron plays on oligodendrocyte function, other aspects of brain maturation, including synaptogenesis, axonal formation, and dendritic sprouting, may also be sensitive to the effects from DCC. Future studies utilizing alternative imaging techniques and analysis strategies are therefore important to understand the overall role that DCC may have on brain development. For example, regional brain volumes derived from anatomical T1-weighted or T2-weighted images could provide insight into whether volumetric differences may exist, while studies utilizing diffusion imaging methodologies may provide new information regarding the underlying white matter microstructure.
Conclusion
At 12-months of age, infants who received DCC had greater myelin content in important brain regions involving motor function, visual/spatial, and sensory processing. A placental transfusion at birth appears to increase myelin content in the early developing brain. DCC is a simple, low-tech, inexpensive technique that appears to make a difference in the developing brains of 12-month-old children, without causing harm. Additional research is needed to examine the longitudinal impact and developmental outcomes related to the timing of umbilical cord clamping and the developing brain.
Supplementary Material
Key Points.
DCC resulted in higher hematocrits in newborn period.
DCC appears to increase myelin at 12 months.
Gender did not influence study outcomes.
Acknowledgments
The authors deeply appreciate the contributions of William Oh, MD, Betty Vohr, MD, and Michael Georgieff, MD for their advice on the study design, Megan Wang for data management, and Cynthia Johnson for administrative support. Thanks are also due to the staff at Women & Infants Hospital and all the parents and their infants for their participation in this study.
Funding
This study was supported by the Bill & Melinda Gates Foundation, Seattle, WA (OPP1061070 [to J.S.M.]); the National Institutes of Health (1R01HD076589 [to J.S.M., D.A.E.-O., S.C.L.D.], R01 MH087510 [to S.C.L.D.], and R00MH110596 [to D.C.D.]). The study sponsors had no role in design, data collection, analyses, interpretation, writing of the manuscript, or decision to submit.
Footnotes
Conflict of Interest
None declared.
References
- 1.Farrar D, Airey R, Law GR, Tuffnell D, Cattle B, Duley L. Measuring placental transfusion for term births: weighing babies with cord intact. BJOG 2011;118(01):70–75 [DOI] [PubMed] [Google Scholar]
- 2.Yao AC, Moinian M, Lind J. Distribution of blood between infant and placenta after birth. Lancet 1969;2(7626):871–873 [DOI] [PubMed] [Google Scholar]
- 3.Arcilla RA, Oh W, Lind J, Gessner IH. Pulmonary arterial pressures of newborn infants born with early and late clamping of the cord. Acta Paediatr Scand 1966;55(03):305–315 [DOI] [PubMed] [Google Scholar]
- 4.Jaykka S Capillary erection and the structural appearance of fetal and neonatl lungs. Acta Paediatr (Stockh) 1958;47(05):484–500 [DOI] [PubMed] [Google Scholar]
- 5.Nelle M, Zilow EP, Bastert G, Linderkamp O. Effect of Leboyer childbirth on cardiac output, cerebral and gastrointestinal blood flow velocities in full-term neonates. Am J Perinatol 1995;12(03): 212–216 [DOI] [PubMed] [Google Scholar]
- 6.Oh W, Lind J. Renal function and blood volume in newborn infant related to placental transfusion. Acta Paediatr Scand 1966; 55:197–210 [Google Scholar]
- 7.Oh W, Lind J. Venous and capillary hematocrit in newborn infants and placental transfusion. Acta Paediatr Scand 1966;55(01): 38–48 [DOI] [PubMed] [Google Scholar]
- 8.Cernadas J, Carroli G, Pellegrini L, Otano L, Ferreira M, Ricci C. The effect of timing of cord clamping on neonatal venous hematocrit values and clinical outcome at term: a randomized controlled trial. Obstet Gynecol Surv 2006;61(09):564–565 [DOI] [PubMed] [Google Scholar]
- 9.Chaparro CM, Neufeld LM, Tena Alavez G, Eguia-Líz Cedillo R, Dewey KG. Effect of timing of umbilical cord clamping on iron status in Mexican infants: a randomised controlled trial. Lancet 2006;367(9527):1997–2004 [DOI] [PubMed] [Google Scholar]
- 10.Andersson O, Hellström-Westas L, Andersson D, Domellöf M. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: a randomised controlled trial. BMJ 2011;343:d7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercer JS, Erickson-Owens DA, Deoni SCL, et al. Effects of delayed cord clamping on 4-month ferritin levels, brain myelin content, and neurodevelopment: a randomized controlled trial. J Pediatr 2018;203:266–272.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connor JR, Menzies SL. Relationship of iron to oligodendrocytes and myelination. Glia 1996;17(02):83–93 [DOI] [PubMed] [Google Scholar]
- 13.Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia 2009;57(05): 467–478 [DOI] [PubMed] [Google Scholar]
- 14.Georgieff MK. The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem Soc Trans 2008;36(Pt 6):1267–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Georgieff MK. Iron assessment to protect the developing brain. Am J Clin Nutr 2017;106(Suppl 6):1588S–1593S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lozoff B, Smith JB, Kaciroti N, Clark KM, Guevara S, Jimenez E. Functional significance of early-life iron deficiency: outcomes at 25 years. J Pediatr 2013;163(05):1260–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr 2003;23:41–58 [DOI] [PubMed] [Google Scholar]
- 18.Barks A, Hall AM, Tran PV, Georgieff MK. Iron as a model nutrient for understanding the nutritional origins of neuropsychiatric disease. Pediatr Res 2019;85(02):176–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercer JS, Erickson-Owens DA, Collins J, Barcelos MO, Parker AB, Padbury JF. Effects of delayed cord clamping on residual placental blood volume, hemoglobin and bilirubin levels in term infants: a randomized controlled trial. J Perinatol 2017;37(03):260–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deoni SCL, Dean DC III, O’Muircheartaigh J, Dirks H, Jerskey BA. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage 2012;63(03):1038–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dean DC III, O’Muircheartaigh J, Dirks H, et al. Characterizing longitudinal white matter development during early childhood. Brain Struct Funct 2015;220(04):1921–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deoni SCL, O’Muircheartaigh J, Elison JT, et al. White matter maturation profiles through early childhood predict general cognitive ability. Brain Struct Funct 2016;221(02):1189–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deoni SCL, Zinkstok JR, Daly E, Ecker C, Williams SC, Murphy DG; MRC AIMS Consortium. White-matter relaxation time and myelin water fraction differences in young adults with autism. Psychol Med 2015;45(04):795–805 [DOI] [PubMed] [Google Scholar]
- 24.Kitzler HH, Su J, Zeineh M, et al. Deficient MWF mapping in multiple sclerosis using 3D whole-brain multi-component relaxation MRI. Neuroimage 2012;59(03):2670–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolind S, Matthews L, Johansen-Berg H, et al. Myelin water imaging reflects clinical variability in multiple sclerosis. Neuroimage 2012;60(01):263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dean DC III, Hurley SA, Kecskemeti SR, et al. Association of amyloid pathology with myelin alteration in preclinical Alzheimer disease. JAMA Neurol 2017;74(01):41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullen E Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Services, Inc; 1995 [Google Scholar]
- 28.Briggs-Gowan M, Carter A. Brief Infant Toddler Social Emotional Assessment Manual. San Antonio: Harcourt Assessment; 2006 [Google Scholar]
- 29.Deoni SCL, Rutt BK, Jones DK. Investigating exchange and multi-component relaxation in fully balanced steady-state free precession imaging. J Magn Reson Imaging 2008;27(06):1421–1429 [DOI] [PubMed] [Google Scholar]
- 30.Heller R, Stanley D, Yekutieli D, Rubin N, Benjamini Y. Cluster-based analysis of FMRI data. Neuroimage 2006;33(02): 599–608 [DOI] [PubMed] [Google Scholar]
- 31.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23(Suppl 1):S208–S219 [DOI] [PubMed] [Google Scholar]
- 32.Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience 2014;276:48–71 [DOI] [PubMed] [Google Scholar]
- 33.Cusick SE, Georgieff MK, Rao R. Approaches for reducing the risk of early-life iron deficiency-induced brain dysfunction in children. Nutrients 2018;10(02):E227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao R, Bora R. Timing of umbilical cord clamping and infant brain development. J Pediatr 2018;203:8–10 [DOI] [PubMed] [Google Scholar]
- 35.Cotten CM, Murtha AP, Goldberg RN, et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr 2014;164(05):973–979.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao Y, Cotten M, Tan S, Kurtzberg J, Cairo MS. Rescuing the neonatal brain from hypoxic injury with autologous cord blood. Bone Marrow Transplant 2013;48(07):890–900 [DOI] [PubMed] [Google Scholar]
- 37.Kc A, Rana N, Målqvist M, Jarawka Ranneberg L, Subedi K, Andersson O. Effects of delayed umbilical cord clamping vs early clamping on anemia in infants at 8 and 12 months: a randomized clinical trial. JAMA Pediatr 2017;171(03):264–270 [DOI] [PubMed] [Google Scholar]
- 38.Andersson O, Domellöf M, Andersson D, Hellström-Westas L. Effect of delayed vs early umbilical cord clamping on iron status and neurodevelopment at age 12 months: a randomized clinical trial. JAMA Pediatr 2014;168(06):547–554 [DOI] [PubMed] [Google Scholar]
- 39.Mercer JS, Erickson-Owens DA. Rethinking placental transfusion and cord clamping issues. J Perinat Neonatal Nurs 2012;26(03): 202–217, quiz 218–219 [DOI] [PubMed] [Google Scholar]
- 40.Boere I, Roest AAW, Wallace E, et al. Umbilical blood flow patterns directly after birth before delayed cord clamping. Arch Dis Child Fetal Neonatal Ed 2015;100(02):F121–F125 [DOI] [PubMed] [Google Scholar]
- 41.Di Tommaso M, Carotenuto B, Seravalli V, et al. Evaluation of umbilical cord pulsatility after vaginal delivery in singleton pregnancies at term. Eur J Obstet Gynecol Reprod Biol 2019; 236:94–97 [DOI] [PubMed] [Google Scholar]
- 42.Winkler A, Gustaffson A, Andersson O. Effects of extended delayed cord clamping on bilirubin and postpartum hemorrhage. Poster presented at: Pediatric Academic Societies; 2018; Toronto, CA [Google Scholar]
- 43.Kc A, Målqvist M, Rana N, Ranneberg LJ, Andersson O; KC A. Effect of timing of umbilical cord clamping on anaemia at 8 and 12 months and later neurodevelopment in late pre-term and term infants; a facility-based, randomized-controlled trial in Nepal. BMC Pediatr 2016;16:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Muircheartaigh J, Dean DC III, Ginestet CE, et al. White matter development and early cognition in babies and toddlers. Hum Brain Mapp 2014;35(09):4475–4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deoni SCL, Kolind SH. Investigating the stability of mcDESPOT myelin water fraction values derived using a stochastic region contraction approach. Magn Reson Med 2015;73(01):161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


