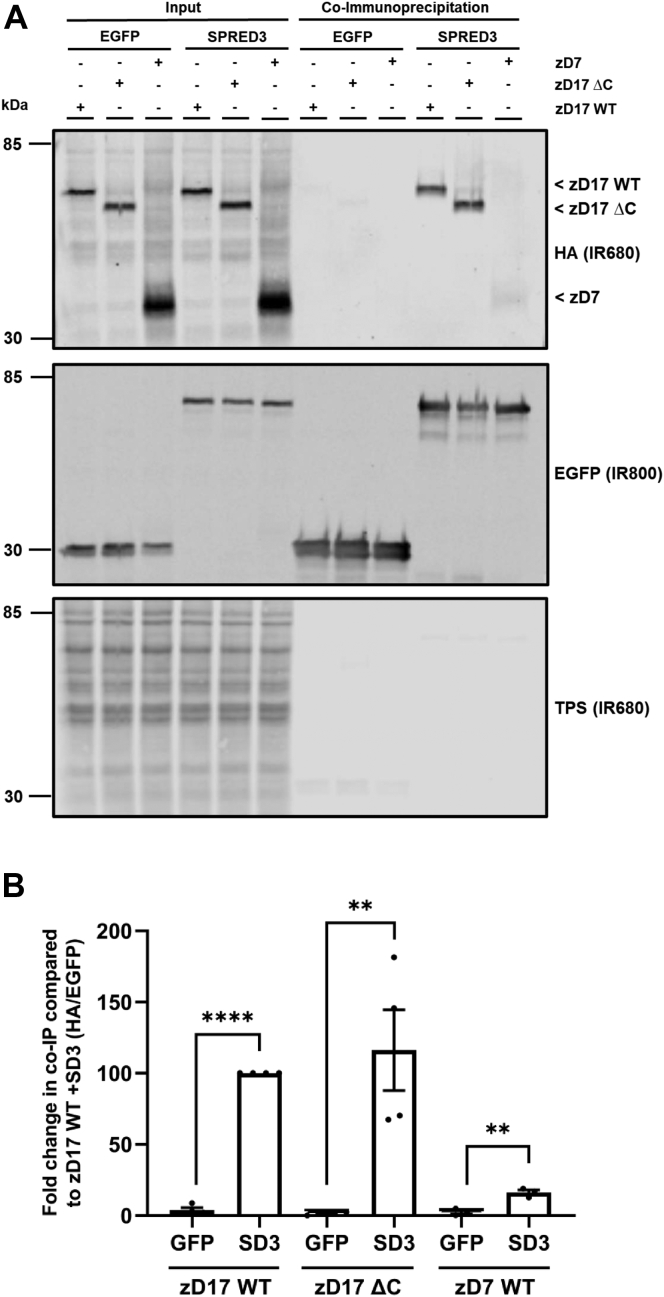
Figure 9.
SPRED3 can bind to a zDHHC17 mutant that lacks the C terminus and displays stronger binding to zDHHC17 than zDHHC7.A, HEK293T cells cotransfected with HA-tagged zDHHC17 WT, zDHHC17 ΔC (C-terminal end removed – see Fig. 7B; zDHHC17 ΔC; aa 11–569), or zDHHC7 WT along with plasmids encoding EGFP-tagged SPRED3 WT or EGFP alone (as a control). Cell lysates were incubated with agarose beads conjugated to an EGFP antibody and coimmunoprecipitated proteins were resolved by immunoblot. Representative images showing coimmunoprecipitated zDHHC17 or zDHHC7 (top; IR680), and EGFP-tagged protein levels (middle; IR800) detected on the same immunoblot. A total protein stain (TPS) is also shown (bottom panel; IR680). The positions of the molecular weight markers are shown on the left side of all immunoblots. B, graph showing the fold change in coimmunoprecipitated zDHHC17 WT, zDHHC17 ΔC, or zDHHC7 WT after normalization. SD3 = SPRED3. Error bars represent ± SEM; each replicate is shown with filled circles. Differences were analyzed by unpaired t test to each EGFP control (∗∗∗∗ denotes p < 0.0001 and ∗∗p < 0.01, n = 4 from three independent experiments).