TABLE 2.
Potential pharmacological targets and related inhibitors targeting fatty acid synthesis.
| Drug target | Notable inhibitors | Inhibitor description | IC50 | Development status | Related diseases | Chemical st8ructure | References |
|---|---|---|---|---|---|---|---|
| SLC25A1 (CIC) | CTPI-2 | — | 3.5 μM | Preclinical Stage | Steatosis, Obesity |

|
Tan et al. (2020) |
| SLC25A1 (CIC) | BTA | First-generation inhibitor | — | Preclinical Stage | Solid cancer |

|
Catalina-Rodriguez et al. (2012) |
| ACLY | Bempedoic acid | An prodrug; Converted to an active drug in liver | 29 uM | FDA approved | Hypercholesterolemia, Mixed dyslipidemia, Statin intolerance |

|
Feng et al. (2020); Masana Marin and Plana Gil, (2021); Ray et al. (2019) |
| ACLY | Hydroxycitric acid | Natural product from Garcinia | — | Phase 4 | Obesity, Diabetes |

|
Jena et al. (2002) |
| ACLY | SB-204990 | — | — | Preclinical Stage | Hypolipidaemic |

|
Feng et al. (2020); Pearce et al. (1998) |
| ACLY | NDI-091143 | High-affinity | 2.1 nM | Preclinical Stage | Thyroid cancer |
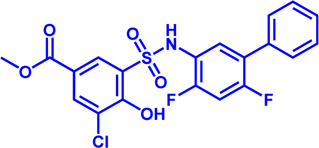
|
Huang et al. (2022); Wei et al. (2019) |
| ACC | ND-630 (Firsocostat) | Reversible, highly specific | — | Phase 1 | Hepatic Steatosis; Obesity |
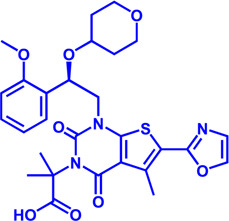
|
Alkhouri et al. (2020) |
| ACC | TOFA | Allosteric inhibitor | — | Preclinical stage | Ovarian cancer; Prostate cancer |
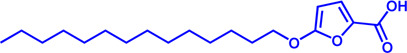
|
Guseva et al. (2011); Li et al. (2013); Wang et al. (2009) |
| ACC | PF-05221304 | Liver-specific | — | Phase 2 | NASH |

|
Ross et al. (2020) |
| ACC1 | soraphen-A | — | — | Preclinical stage | Prostate cancer; High-Fat Diet-induced Insulin Resistance, Hepatic Steatosis |

|
Beckers et al. (2007) |
| FASN | Cerulenin | Natural inhibitor from Cephalosporium caeruleus | — | Preclinical Stage | Hepatic Steatosis; Solid cancer |

|
Currie et al. (2013); Menendez and Lupu, (2007) |
| FASN | C75 | Synthetic analog of cerulenin | 35 μM | Preclinical Stage | Prostate cancer |
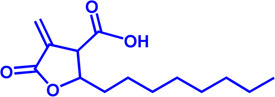
|
Shimokawa et al. (2002); Thupari et al. (2002) |
| FASN | EGCG | An phenolic antioxidant from plants such as green tea | — | Phase 2 | A wild range of cancers |
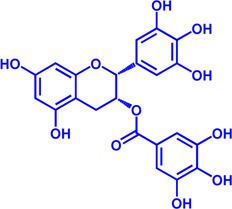
|
Humbert et al. (2021); Khan et al. (2006) |
| FASN | Orlistat | The saturated derivative of lipstatin | — | Phase 3 | Obesity |

|
Pemble et al. (2007) |
| FASN | TVB-2640 Denifanstat | Reversible | 0.052 μM | Phase 3 | NAFLD; Solid Malignant Tumors |

|
Loomba et al. (2021) |
| FASN | IPI-9119 | Selective and Irreversible | 0.3 nM | Preclinical Stage | Castration-resistant prostate cancer |

|
Zadra et al. (2019) |
| FASN | TVB3664 | Reversible | 18 nM | Preclinical Stage | Colorectal cancer |

|
Wang et al. (2022a) |
| SCD1 | A939572 | Synthetic Inhibitor | 37 nM | Preclinical Stage | Renal cell carcinoma |

|
Leung and Kim, (2013); von Roemeling et al. (2013) |
| SCD1 | MK-8245 | Liver-selective inhibitor | 1 nM | Phase1 clinical trials (NCT00790556) for T2D | Diabetes and Dyslipidemia |

|
Oballa et al. (2011) |
| SCD1 | CAY10566 | — | 26 nM | Preclinical Stage | Breast cancer, Lung cancer, Colorectal cancer |

|
Liu et al. (2007) |
| DGAT1 | AZD-7687 | Selective | 80 nM | Phase1 | Type 2 Diabetes, Obesity |

|
Morentin Gutierrez et al. (2019) |
| DGAT1 | AZD3988 | — | 6 nM | Preclinical stage | Type 2 Diabetes, Obesity |
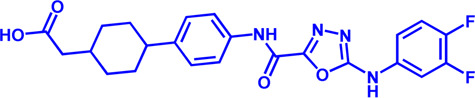
|
McCoull et al. (2012) |
| DGAT1 | PF-04620110 | selective | 19 nM | Phase1 | Type 2 Diabetes, Obesity |

|
Dow et al. (2011), Lee et al. (2013) |
| DGAT1 | A922500 | selective | 9 nM | Preclinical stage | hyperlipidemia |
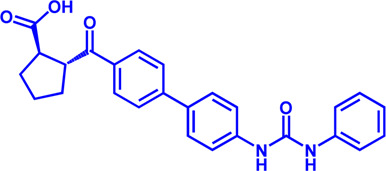
|
Cheng et al. (2020), King et al. (2010) |
| DGAT2 | PF-06424439 | selective | 14 nM | Preclinical stage | hyperlipidemia |

|
Futatsugi et al. (2015) |
| DGAT2 | PF-06865571 (Ervogastat) | well-tolerated | — | Phase1 | NASH, NAFLD |

|
Calle et al. (2021) |
| DGAT2 | JNJ DGAT2-A | selective | — | Preclinical stage | Type 2 Diabetes, Solid cancer |

|
Irshad et al. (2017) |
