TABLE 3.
Potential pharmacological targets and related inhibitors targeting cholesterol synthesis.
| Drug target | Notable inhibitors | IC50 | Development status | Corresponding diseases | Chemical structure | References |
|---|---|---|---|---|---|---|
| HMGCR | Mevastatin | 1 nM | Upgraded to lovastatin | Hyperlipemia; Coronary Heart Disease |

|
Glynn et al. (2008) |
| HMGCR | Lovastatin | 3.4 nM | FDA approved | Hypercholesterolemia |

|
Mulder et al. (2015), Zeller and Uvodich, (1988) |
| HMGCR | Pravastatin | 5.6 μM | FDA approved | Cardiovascular Disease |

|
McTavish and Sorkin, (1991) |
| HMGCR | Simvastatin | 95.6 μM | FDA approved | Hypercholesterolemia; Hypertriglyceridemia |

|
Gryn and Hegele, (2015) |
| HMGCR | Fluvastatin | 8 nM | FDA approved | Hypercholesterolemia |
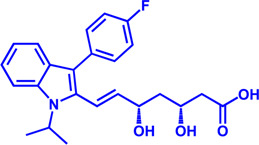
|
Scripture and Pieper, (2001) |
| HMGCR | Rosuvastatin | 11 nM | FDA approved | Hypertriglyceridemia |
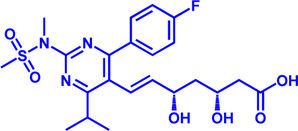
|
Davidson, (2004), Olsson et al. (2002) |
| HMGCR | Pitavastatin | 5.8 nM | FDA approved | Dyslipidemia; Hypercholesterolemia |

|
Chan et al. (2019), Hoy, (2017) |
| HMGCR | Atorvastatin | 154 nM | FDA approved | Hypercholesterolemia; Dyslipidemias |

|
Hu et al. (2021b) |
| HMGCR | Cerivastatin | 6 nM | Withdrawn from the market due to a high risk of rhabdomyolysis | Hypercholesterolemia; Dyslipidemia |
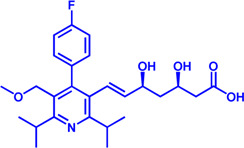
|
Bischoff et al. (1997), Furberg and Pitt, (2001) |
| ACAT1 | K-604 (selective) | 0.45 μM for ACAT1; 102.85 μM for ACAT2 | Phase 2 Completed | Atherosclerosis |

|
Ikenoya et al. (2007) |
| ACAT1 | Nevanimibe (selective) | 52 nM | Discontinued - Phase-II | Adrenocortical Carcinoma; Congenital adrenal Hyperplasia; Cushing syndrome |
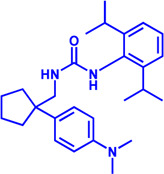
|
El-Maouche et al. (2020), Long et al. (2020) |
| ACAT | CI 976 | 0.073 μM | preclinical | Atherosclerosis; Hyperlipidaemia |

|
Krause et al. (1993) |
| ACAT | Avasimibe (CI-1011) | 3.3 μM | Discontinued - Phase-III | Atherosclerosis; Hyperlipidaemia |

|
Llaverias et al. (2003), Schmidt et al. (2021), Zhou et al. (2022) |
| ACAT | RP-64477 | 503 nM, in human hepatic (HepG2) | phase II | Hyperlipidemia |
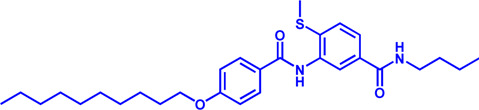
|
Bello et al. (1996) |
| ACAT | Eflucimibe | 39 nM for ACAT1; 110 nM for ACAT2 | Phase-II discontinued | Atherosclerosis; Hyperlipidemia |

|
Lopez-Farre et al. (2008) |
| ACAT | Cyclandelate | 80 μM (Rat hepatic ACAT) | Not approved in U.S. or Canada; Approved in Europe | Arteriosclerosis |

|
Heffron et al. (1990) |
