TABLE 4.
Potential pharmacological targets and inhibitors targeting FAO.
| Drug target | Notable inhibitors | Inhibitor description | IC50 | Development status | Related diseases | Chemical structure | References |
|---|---|---|---|---|---|---|---|
| ACSLl | Triacsin C | An natural inhibitor, from Streptomyces aureofaciens | 6.3 uM | Preclinical stage | Lung cancer; Colon cancer; Stomach cancer; Brain cancer; Breast cancer |

|
Mashima et al. (2005) |
| CPT1 | Etomoxir | Irreversible; Malonyl-CoA mimetic | 5–20 nM (rat liver) | Phase II clinical trial stopped due to hepatoxicity | Leukemia; Glioblastoma |

|
Bristow, (2000); Divakaruni et al. (2018); Lopaschuk et al. (1988); O'Connor et al. (2018) |
| CPT1, CPT2 | Perhexiline (Pexsig) | Inhibit CPT1; to a lesser extent, CPT2 | 77 μM (rat heart CPT1); 148 μM (CPT1A) | Used primarily in Australia and New Zealand Adverse effects: nausea, hypoglycemia, neuropathy, and hepatitis | Severe angina pectoris |

|
Ashrafian et al. (2007); Ren et al. (2020) |
| CPT1 | ST1326 (Teglicar) | Amino-Carnitine derivative; highly selective for CPT1A; Reversible | 0.68 μM (CPT1A) | Discontinued - Phase-II for Type-2 diabetes | Diabetes; Neurodegenerative diseases including Huntington’s disease |

|
Bertapelle et al. (2022); Conti et al. (2011) |
| CPT1 | 2-tetradecylglycidate (TDGA) | Glycidic acid analog; An oxirane carboxylate inhibitor | — | Preclinical Stage, (induce myocardial hypertrophy) | Diabetes |
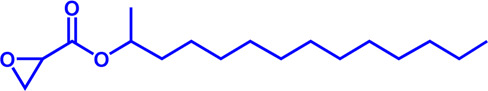
|
Obici et al. (2003); Schlaepfer and Joshi, (2020); Wolkowicz et al. (1999) |
| CACT | EN936 (SLC25A20-IN-21) | — | — | Preclinical Stage | — |

|
Parker et al. (2017a); Parker et al. (2017b) |
| VLCAD | Avocadyne | — | — | Phase 1 | Acute Myeloid Leukemia; Hyperglycemia |

|
Tcheng et al. (2021a); Tcheng et al. (2022); Tcheng et al. (2021b) |
| TFPβ | Ranolazine | — | — | FDA approved (NDA #021526) | Chronic Angina |
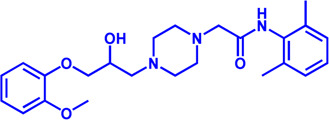
|
Samudio et al. (2010); Sekine et al. (2022) |
| TFP | Trimetazidine | — | 75 nM | Phase 2 (NCT03273387) | Precapillary pulmonary hypertension; Muscle wasting (cachexia) |

|
Gatta et al. (2017) |
