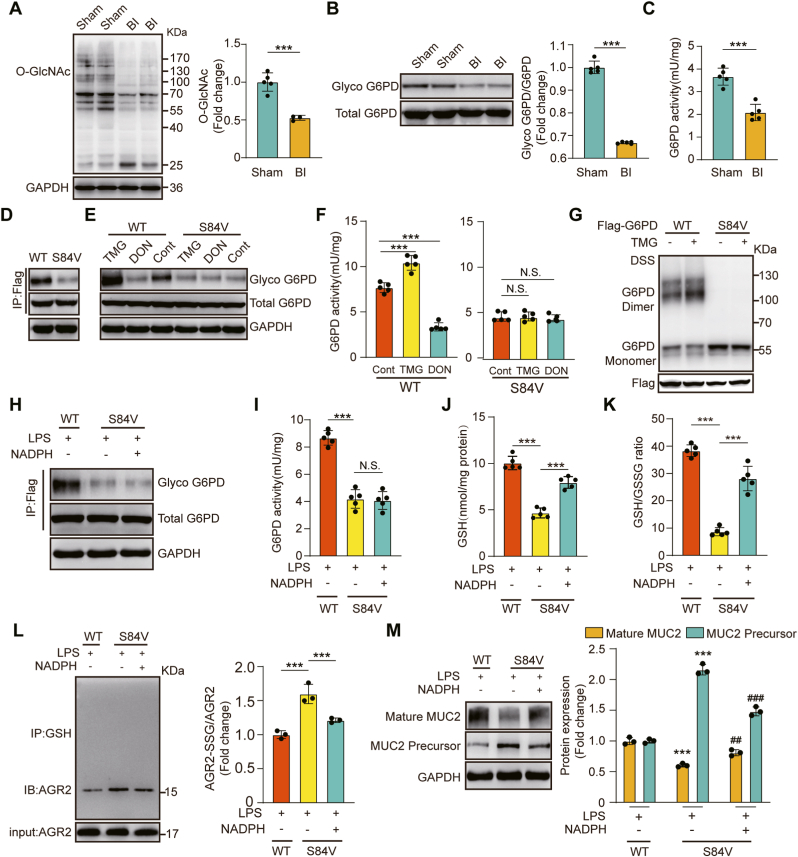
Fig. 6.
O-GlcNAcylation of G6PD regulates its activity through dimerization to promote MUC2 maturation.
(A) Immunoblot analysis of protein O-GlcNAc modification in the distal colons of sham and burn-infected mice at 5 days post-injury (N = 5 per group). (B) Detection of O-GlcNAcylation of G6PD in the distal colons of sham and burn-infected mice by using chemical enzyme labeling and biotinylation (N = 5 per group). (C) G6PD enzyme activity assay (N = 5 per group). (D) Labeling of G6PD glycosylation levels in WT and S84V G6PD cells. (E) Immunoblot for O-GlcNAcylation of G6PD. Cells labelled WT and S84V were treated with DON (20 μM) and TMG (50 μM). (F) G6PD enzyme activity assay of cells under different treatments (N = 5 per group). (G) Oligomerization status in WT and S84V G6PD cells measured by immunoblotting. Cross-linking with 1 mM DSS. (H) Immunoblot for O-GlcNAcylation of G6PD. WT and S84V G6PD cells were exposed to LPS (100 ng/mL) for 6 h and treated with or without NADPH. (I) G6PD enzyme activity assay under different treatments (N = 5 per group). (J) GSH levels in cell lysates (N = 5 per group). (K) GSH/GSSG ratio in cell lysates (N = 5 per group). (L) Co-IP showing S-glutathionylated AGR2 in cell lysates (N = 3 per group). (M) Immunoblot detection of mature MUC2 and immature MUC2 (N = 3 per group). * Compared with the WT group, # Compared with the S84V group without NADPH, #P < 0.05; # #P < 0.01; # # #P < 0.001.